Boron for Prevention of the Spread of Radiation
Boron (B₁₀), which comprises about 20% of elemental boron, is one of the best neutron absorbers. It is dissolved in the cooling water of power plants to control the chain reaction, to absorb enough neutrons to remove excess reactivity in PWR plants. In both BWRs and PWRs, it is used in control rods to control reactivity and change the power shape of the core. Boron and Chernobyl – it was used successfully to help control fallout in the Chernobyl disaster.

Boron and Chernobyl
Neutron detectors often contain boron. The B10 absorbs a neutron emitting alpha, and the ionization caused by the alpha is measured (either in counts or current).
Other reactor designs can start with very little excess reactivity (excess reactivity = capacity to produce more neutrons than required for balance). They can use thermal expansion/contraction as their primary tool to regulate neutron production (negative temperature coefficient). One such type of reactor is the MSR (Molten Salt Reactor). These reactors don’t use boron in regular operation but only use it in an emergency to shut the reactor down quickly.
MSRs can use lower enrichment fuel and produce less power (nuclear waste) than typical reactors.
Boron is used in reactor control rods and injected into the coolant when much neutron absorption is needed.
Thorium also helps reduce Boron/Gadolinium requirements.
In standard reactors (thermal spectrum ones), using Thorium in the place of U238 as the fertile of a reactor flattens the power curve over time, reducing the fissile level needed at reactor startup (typically U235 or Pu239). This is due to U238->Pu239 thermal fission producing an effective 1.9 neutrons per neutron consumed, while Th232->U233 thermal fission produces an effective 2.45 neutrons per neutron consumed.
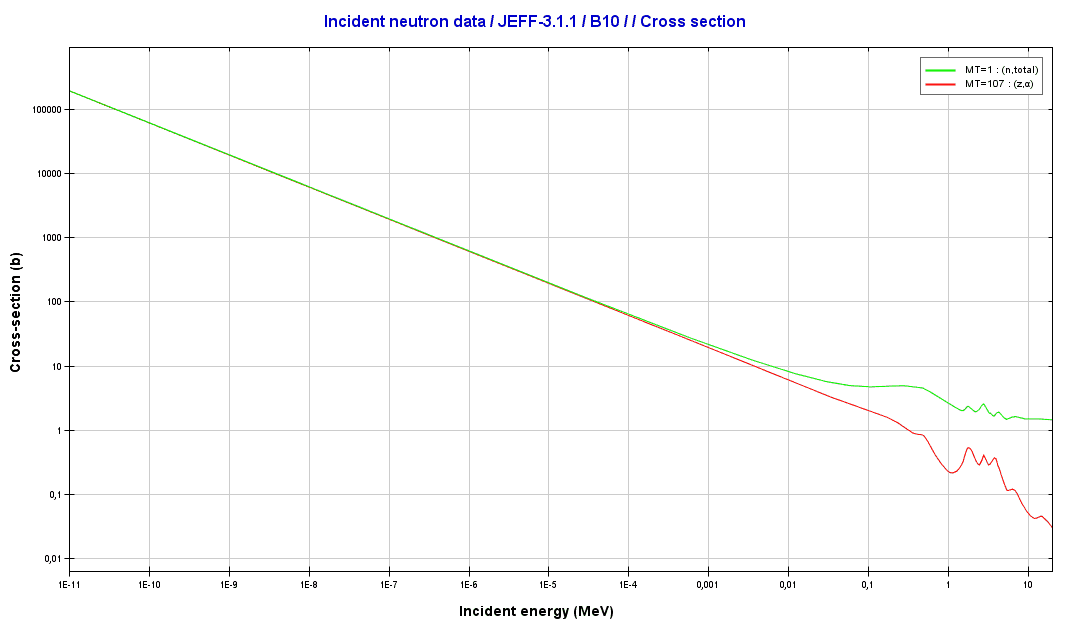
Boron Data
Source – B₁₀ graph in respect to reaction cross-section versus Incident Energy of B₁₀
Boron’s Role in Nuclear Reactors
Boron is used as a neutron poison because of its neutron-absorbing properties. Absorbing neutrons is vital to control the excess reactivity of a reactor core due to having more mass at the beginning of life than needed for criticality.
Boron can be present in a nuclear core in a few ways. It can be added to the reactor coolant in boric acid. It can also be built into the core in boron carbide assemblies to control neutron flux at certain places in a reactor core to try to even out the flux that may be misshapen from control rods.
Adjusting boron concentration is just like adjusting control rod height. Each has the effect of changing core temperature. As the fuel burns out, boron concentration is lowered by adding water to the coolant to dilute the mix. Otherwise, the temperature will fall as the fuel burns out.
Neutron Absorber
Boron is used as a neutron poison because of its neutron-absorbing properties. Absorbing neutrons is essential to control the excess reactivity of a reactor core due to having more mass at the beginning of life than needed for criticality.
Boron can be present in a nuclear core in a few ways. It can be added to the reactor coolant in the form of boric acid. It can also be built into the core in boron carbide assemblies in order to control neutron flux at certain places in a reactor core to try to even out the flux that may be misshapen from control rods.
Adjusting boron concentration is just like adjusting control rod height. Each has the effect of changing core temperature. As the fuel burns out, boron concentration is lowered by adding water to the coolant to dilute the mix. Otherwise, the temperature will fall as the fuel burns out.
How Boron Helps Control Nuclear Radiation
Because of its radiation-absorbing effect, boron is widely used in nuclear reactors’ shielding, control, and safety systems of nuclear reactors.
- Control rods Boron: Boron, a primary neutron absorber, is used to construct control rods within a nuclear reactor’s core.
- Shielding: Boron is an additive to steel that absorbs neutron radiation better. Boron-based steel is used for shielding and construction throughout the plant. Plastics throughout the plant are also treated with boron.
- Coolant water: Borated Water is often used to remove heat from the reactor core. Borates can be added to water to help control the fission rate. You can adjust the level of boron in the water to alter the amount of reactivity in the plant.
- On-site: Nuclear plants often keep large amounts of boron. If a problem arises, the stored boron can help coolant water and ensure shutdowns if necessary.
Most of the (n,alpha) reactions of thermal neutrons are 10B(n,alpha)7Li reactions accompanied by 0.48 MeV gamma emission.
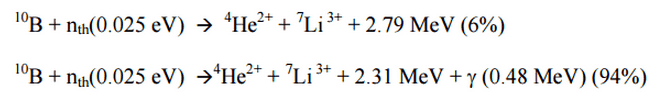
Boron Neutron Reaction
Source – B₁₀ Nuclear power reaction
Boron is essential to nuclear power safety due to several reasons. Boron is vital in the production and refinement of uranium hexafluoride. Research has shown that it can increase the neutron capture cross-section by two orders. Its ability to slow down neutrons means it is an ideal element for studying how to manage radioactive materials.
This function is important, but it also stops recombination reactions that could create magnetic fields. These can potentially cause reactor shutdowns.
Typical Uses for Boron in Nuclear Power Projects
Isotope 10B has a wide range of applications. Its reaction cross-section for thermal neutrons (n, alpha) is about 3840 barns (for 0.025 eV neutrons), making it a perfect neutron absorber. Natural boron is primarily used in nuclear power, but it is not an exception to enriched boron (by the isotope 10B). It is used as a neutron absorber in the following applications:
- Chemical shim. Chemical shim refers to the dissolution of boric acid in the coolant/modulator. It is used to control reactivity over long periods.
- Control rods. Isotope 10B is often used as a neutron-absorbing material in control rods.
- Safety systems. Nuclear reactor safety systems have one of their primary objectives shutting down the reactor and keeping it shut down. As a result, all safety systems (designed to ensure the reactor’s subcritical operation after the transient) use high concentrations of boric acid.
- Burnable absorbers. Isotope 10B is widely used as a burnable absorbent. Compared with gadolinium absorbers (another commonly used durable material), 10B has a much smaller cross-section. Thus, 10B compensates for reactivity for a more extended period, but not in such a heavy way.
- A converter in neutron detectors. As neutrons have no ionizing properties, they must first be converted into charged particles before being detected. Among the neutron converter materials, 10B is most commonly found.
Case Study – Boron Helps Stop Nuclear Radiation in Chernobyl Disaster
On April 26, 1986, Unit 4 of the former Soviet Union’s nuclear power station at Chernobyl was destroyed by an unexpected surge of power. The environment was exposed to large amounts of radioactive material due to the following fire and accident.
During safety testing on the steam turbine for an RBMK type nuclear reactor, the accident occurred. The power output dropped unexpectedly to zero during a planned reduction in reactor power as preparation for the test. Operators could not restore the required power level, which caused the reactor to become unstable. The operating instructions did not mention this risk, so operators continued the test.
After the test was completed, the operators activated a reactor shutdown. Operator negligence and critical design flaws led to the reactor being set up for an explosion. Instead of the reactor being shut down, an uncontrolled nuclear chain reaction started, releasing vast amounts of energy.

Liquidators clean the roof of reactor 3. In the beginning, workers tried clearing the radioactive debris using West German, Japanese and Russian robots but they could not cope with the extreme radiation levels and so the authorities decided to use humans. Employees could not stay any longer than 40 seconds any one time, before the radiation dose they received was the maximum authorized dose a human being should receive in his entire life. Many liquidators have since died or suffer from severe health problems. (Photo by Igor Kostin/Sygma via Getty Images)
Image Source: the atlantic.com
Boron and Sand Used at Chernobyl Disaster
Boron and sand were used to stop nuclear reactor number four at the Chernobyl Nuclear Power Plant. As a result of the helicopter drop, 5000 metric tons of sand, clay, and neutron-absorbing boron were dropped on the reactor.
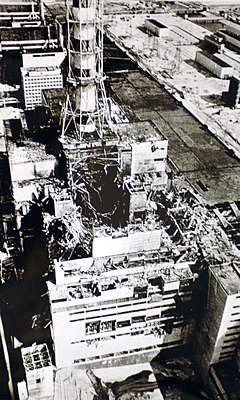
Chernobyl Reactors
Source: Wikipedia
A large fire raged in the ruins of the No. 4 reactor of the Chernobyl Nuclear Power Plant in 1986. Overrun by radiation victims, the local hospital in Pripyat suffered a severe shortage of resources. Deadly radioactive dust had drifted out of the Soviet Union and into Sweden. The air above the reactor glowed where the uranium core had become exposed. And the people leading the disaster response decided to dump thousands of tons of sand and boron on the core.
According to Kathryn Huff, a nuclear reactor engineer at the University of Illinois at Urbana-Champaign, the exposure of a nuclear core to the atmosphere poses a problem at least on two levels. The first is the ongoing nuclear-fission reactions. The neutrons that uranium emits are being slammed into other elements of uranium and splitting them. These uranium atoms are producing more energy, feeding the entire hot mess. This non-contained reaction also releases incredible amounts of radiation that pose a grave danger to anyone who comes in contact with it.
Another problem, which is much more severe, is releasing lots of smoke, dust, and debris from the fire into the atmosphere. The nuclear reactor produced a lot of this gunk, which mattered from the core. As a result, this includes various lightweight elements (or isotopes) that are formed when uranium molecules split.
When the problem arose, sand and boron were dumped (the Chernobyl mixture included clay and lead). The sand covered the reactor and suffocated it, creating a deadly smoke plume. The boron could, theoretically, squelch nuclear power.
Nucelar Power Safety
For nuclear power safety, research into neutrons can help resolve measurement problems. Understanding how neutrons are absorbed is crucial. The reactor operators can control the process when it is time for borates to be used on-site at their plants.
The safety of nuclear power plants is essential in our modern world. We can continue to explore the possibilities of discovery and use of this resource. But we need to be aware of how any findings might affect current practices. Judicious use of Boron helps make a safer plant environment with lower radiation risk even with release into the atmosphere and/or groundwater.