Boron – Anything but Boring
In this podcast from 2010, Pat Bailey covers Boron in Chemistry World in a podcast revealing all about Boron. Here are some extracts from the fascinating analysis into Boron:
Click the image below to listen to a Scientist’s admiration for Boron (podcast first broadcast in 2010) on Chemistry World. For other information on how Boron is recently becoming even more ‘sexy’. See how Boron is helping the world reach a low carbon future in Borates Today.
Boron Redux
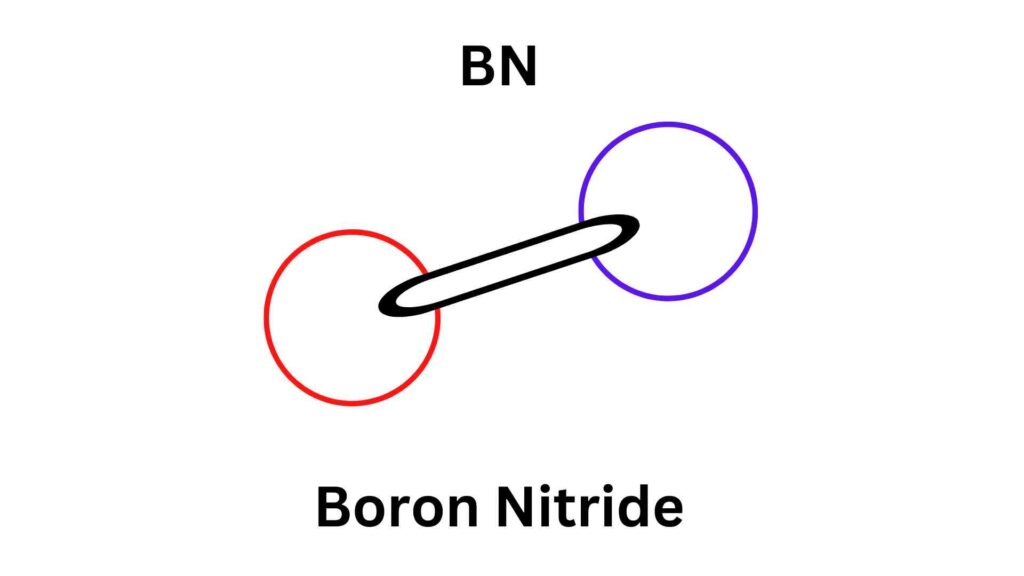
“Let’s start with the boring bit. Boron is usually isolated as a brown, amorphous solid. I don’t know anyone who thinks the element boron has anything interesting. But its unexpected side starts to emerge when you look at some simple compounds of boron. Consider the nitride, for example – just the two elements at numbers five and seven in the periodic table, but able to join forces to provide hard diamond or soft graphite-like structures, very similar to those of the sixth element, carbon.
“Then there is the trifluoride – remember that acids were first classified as substances that could provide protons, but BF3 is the archetypal Lewis acid, which doesn’t have a proton in sight, yet can coordinate with lone pairs, allowing it to catalyze an array of reactions. It can achieve this chemistry because boron has two sides – it is set up to form three bonds with adjacent atoms, but even in this state, it readily forms an extra bond to complete the second main shell of eight electrons. But when it does this, it acquires a negative charge, and it can only regain neutrality by losing one of its bonds – it does have a split personality.
“But the real interest – the ‘skydiving’ – starts when we look at the trihydride of boron. We’ll return to this later on, as BH3 has structural subtleties that will really take us into sexy territory. But at this stage we’ll simply see how boron’s schizophrenic side can be used to good effect: add BH3 to an alkene, then throw in some alkaline hydrogen peroxide, and the oxygen first attaches to the boron, and then gets shuttled onto the adjacent carbon, all driven by this balance between 3- and 4-valent boron.
This rather complicated reaction (mechanistically) is very reliable, and has been used for decades now as a simple way of turning alkenes into alcohols. Building on this idea, lots of clever variants allow one to introduce the alcohol very selectively, including my favourite of the reagent made by reacting borane with cycloocta-1,5-diene; the resulting dialkylborane is incredibly selective at attacking only the least substituted carbon of an alkene, and its often abbreviated schematically to a BH unit hanging down from two arcs, leading to its nickname as the parachute molecule.”
Boron Today
Since this audio podcast was made, the role of Boron has become much more front of the stage. Boron is now at the heart of tackling global warming, decarbonization, and food security, among other uses and applications.
Borates Today has the initiative to bring awareness of Boron to the forefront of our thinking: We have only one planet. Resources are finite, and it is our joint responsibility to take action to make the world a better place for future generations. Planet Boron is an initiative by Borates Today to harness the strengths and benefits of one of the world’s most precious resources – BORON – and publish news and events about boron’s availability, applications, benefits for achieving the collective vision and shared goals as we embrace multiple goals related to four key areas: Decarbonization; Advanced Energy, Food Security, and Micronutrients.