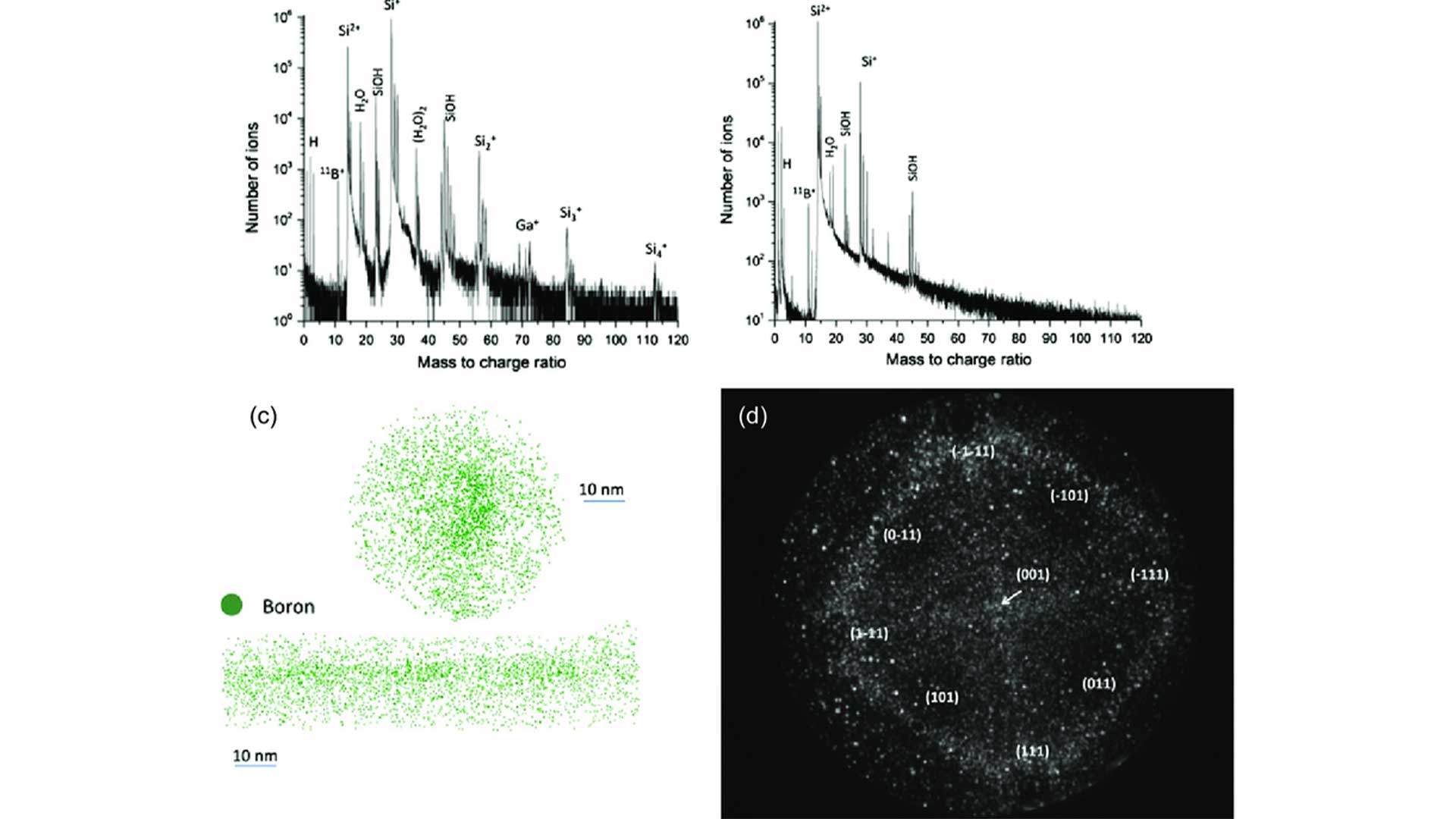
The Boron Mass Spectrum: Isotopes, Peaks, and Real-World Applications
Boron is an element with two stable isotopes: Boron-10 and Boron-11. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. In this article, we will explore the isotopes of boron, the principles of mass spectrometry, and the real-world applications of boron mass spectrometry.
Isotopes of Boron
Boron-10
Boron-10 is the most abundant isotope of boron, accounting for approximately 19.9% of naturally occurring boron. It has a relative isotopic mass of 10 and consists of five protons and five neutrons in its nucleus.
Boron-11
Boron-11 is the other stable isotope of boron, making up about 80.1% of naturally occurring boron. It has a relative isotopic mass of 11 and consists of five protons and six neutrons in its nucleus.
Principles of Mass Spectrometry
Source: Research Gate
Mass spectrometry is an analytical technique used to determine the composition, isotopic abundance, and molecular structure of a sample. Understanding the principles of mass spectrometry is essential to comprehend the mass spectrum of boron.
Mass spectrometry involves ionization, mass analysis, detection, and data analysis. It starts with the ionization of the sample, followed by the separation of ions based on their mass-to-charge ratio (m/z), detection of ions, and analysis of the obtained data.
Let’s break down the key steps involved in mass spectrometry using boron-10 and boron-11 as examples.
Ionization Process
The ionization process converts neutral atoms or molecules in the sample into charged ions. Various methods, such as electron ionization or electrospray ionization, can be used to ionize the sample.
Ionization of the Sample
The first step in mass spectrometry is the ionization of the sample. This process converts neutral atoms or molecules in the sample into charged ions, which can be manipulated and analyzed. Various ionization methods can be used, and I’ll explain two common ones:
Electron Ionization (EI):
In EI, high-energy electrons are used to ionize the sample. Here’s how it works with boron-10 and boron-11:
A sample containing boron-10 and boron-11 atoms is introduced into the mass spectrometer.
Inside the ionization source, the sample is bombarded with high-energy electrons. These electrons have enough energy to remove one or more electrons from the atoms in the sample.
As a result, boron-10 and boron-11 atoms lose one or more electrons and become positively charged ions. These ions now have a net positive charge.
The process can be represented as follows: Boron-10 (10B) -> Boron-10 Ion (10B+) Boron-11 (11B) -> Boron-11 Ion (11B+)
Electrospray Ionization (ESI):
ESI is commonly used for ionizing larger molecules such as peptides or proteins. It’s particularly useful in liquid chromatography-mass spectrometry (LC-MS) applications.
In ESI, a liquid sample containing boron-10 and boron-11 molecules is introduced into the mass spectrometer.
A high-voltage source is applied to create a fine aerosol spray of the sample.
As the liquid droplets evaporate in the electric field, ions are formed. These ions can be positively or negatively charged, depending on the conditions.
In the case of boron-10 and boron-11, the resulting ions may have different charge states, but they are typically in the form of molecular ions (e.g., [B10Hx]+ and [B11Hy]+).
Mass Analysis
After ionization, the ions are separated based on their mass-to-charge ratio using a mass analyzer. Different types of mass analyzers, such as magnetic sectors, quadrupoles, and time-of-flight analyzers, can be used for this purpose.
The mass spectrum obtained from the detector provides valuable information about the composition of the sample. By analyzing the intensities and m/z values of the peaks in the spectrum, researchers can identify the compounds present, determine their relative abundances, and even quantify their concentrations.
For example, in a TOF analyzer, ions are accelerated by an electric field and then travel through a flight tube. The time it takes for ions of different masses to reach the detector at the end of the flight tube is used to determine their m/z values.
Detection and Data Analysis
Detection and Data Analysis
The separated ions are then detected, and their relative abundances at different m/z values are measured. The detected ions are converted into electrical signals and processed to generate a mass spectrum. Data analysis techniques are used to interpret the mass spectrum and extract valuable information about the sample.
Mass Spectrum
The mass spectrum represents the distribution of ions based on their m/z values. Each peak in the mass spectrum corresponds to a specific m/z value, and the position and intensity of the peaks provide insights into the isotopic composition and relative abundance of elements in the sample.
Peaks and Isotopes
In boron mass spectrometry, the peaks in the mass spectrum represent the different isotopes of boron. The relative intensities of these peaks reflect the relative abundances of Boron-10 and Boron-11 isotopes in the sample.
| Isotope | Relative Abundance | Intensity in Mass Spectrum |
|---|---|---|
| Boron-10 | 19.9% | 23 |
| Boron-11 | 80.1% | 100 |
Analyzing Peaks in Boron Mass Spectrum
Analyzing the peaks in the mass spectrum of boron allows for the determination of the relative abundance of each isotope and the calculation of the relative atomic mass of boron.
Relative Abundance Calculation
The relative abundance of each isotope is determined by measuring the intensity of the corresponding peaks in the mass spectrum. The relative abundance of an isotope is proportional to the intensity of its peak.
Determining Relative Atomic Mass
The relative atomic mass of boron is calculated by taking the weighted average of the masses of its isotopes, considering their relative abundances. The mass of each isotope is multiplied by its relative abundance, and the results are summed to obtain the relative atomic mass.
Example Calculation
For example, if the intensity of the Boron-10 peak is 23 and the intensity of the Boron-11 peak is 100, the relative abundance of Boron-10 and Boron-11 can be calculated. Based on these values, the relative atomic mass of boron can be determined.
Applications of Boron Mass Spectrometry
Boron mass spectrometry has various applications in different scientific fields, including chemistry, biology, materials science, and industry.
Chemistry and Material Science
Boron mass spectrometry is used in analyzing chemical reactions, characterizing materials, and studying the behaviour of boron compounds. It provides insights into the composition, structure, and properties of boron-containing compounds. For example, a study published in the Journal of Analytical Atomic Spectrometry utilized boron mass spectrometry to analyze the reaction kinetics of boron-containing compounds in a catalytic system.
Biological and Environmental Research
In biological and environmental research, boron mass spectrometry aids in the study of biological systems, including the detection and identification of metabolites and biomarkers. It also contributes to environmental analysis, helping to understand the distribution and behaviour of boron in the environment. A research article published in the Journal of Mass Spectrometry demonstrated the application of boron mass spectrometry in identifying boron-containing metabolites in biological samples.
Industrial Applications
In industrial sectors, boron mass spectrometry is applied in various ways. For example, in semiconductor manufacturing, it is used to analyze and control the boron concentration in silicon wafers, a critical parameter in semiconductor device fabrication. In the field of nuclear energy, boron mass spectrometry is employed to monitor and analyze boron isotopes in nuclear reactors. A study conducted by researchers at the National Institute of Standards and Technology (NIST) utilized boron mass spectrometry to analyze the boron concentration in silicon wafers used in the production of solar cells.
Analyzing Boron Isotopes in Archaeology
Archaeologists who specialize in studying ancient civilizations and their use of natural resources may use boron isotpoes. For example, in excavation projects, a burial site belonging to a prehistoric society with human remains, may contain artefacts made from a unique material that can be tested as boron-based.
By analyzing the isotopic composition of the artefacts using mass spectrometry, samples from different artefacts can be prepared for analysis. With the help of a mass spectrometer, a boron mass spectrum may reveal the presence of two distinct peaks.
Further analysis of the two peaks as corresponding to the isotopes Boron-10 and Boron-11 can reveal the relative abundance of each isotope based on the peak intensities and determine that the artefacts have a higher percentage of Boron-10 compared to naturally occurring boron.
A conclusion may be drawn that prehistoric society had a specific method of extracting and processing boron, resulting in a unique isotopic composition in the artefacts. Insights into their technological advancements and trade networks may be the conclusion of such research.
In other words, boron mass spectrometry in archaeology can be used to uncover valuable information about the ancient society’s material culture, manufacturing techniques, and potentially their geographical origins. This demonstrates the wide-ranging applications of boron mass spectrometry beyond the traditional fields of chemistry and physics.
Resources and References
For further exploration of boron mass spectrometry, the following resources provide valuable information and references:
Chemistry LibreTexts
The Chemistry Libre Texts website offers comprehensive resources on mass spectrometry, including boron mass spectra. It provides in-depth explanations, examples, and additional references.
National Institute of Standards and Technology (NIST)
The National Institute of Standards and Technology (NIST) offers data on boron trifluoride and boron triiodide mass spectra through subscription sites. These data sources provide valuable information on the mass spectra of these boron compounds.
Scientific Journals
Scientific journals, such as PubMed, publish advanced research articles on boron mass spectrometry. These journals contain in-depth studies, methodologies, and applications of boron mass spectrometry in various scientific disciplines.
FAQ
What is the boron mass spectrum used for?
The boron mass spectrum is used to analyze the isotopic composition of boron samples.
Who can benefit from studying the boron mass spectrum?
Scientists, researchers, and chemists studying boron can benefit from analyzing its mass spectrum.
How does boron mass spectrum analysis work?
Boron mass spectrum analysis involves ionizing boron atoms and separating them by their mass-to-charge ratio.
What are the applications of the boron mass spectrum?
Boron mass spectrum has applications in geology, materials science, environmental monitoring, and nuclear research.
What is the objection to using the boron mass spectrum?
Some may argue that boron mass spectrum analysis is time-consuming and requires specialized equipment.
How accurate is boron mass spectrum analysis?
Boron mass spectrum analysis is highly accurate in determining the isotopic composition of boron samples.