Two-Dimensional Boron Monosulfide Nanosheets
Research into Boron monosulfide nanosheets provides fascinating insights into how they might be applied to make more efficient energy sources in applications including batteries, catalysts, and electronic devices. Recent research shows that two-dimensional (2D) boron monosulfide nanosheets (BS) are reported to possess several stable phases. They showcase unique electronic structures, giving them exciting superconducting, thermoelectric properties, and may be useful for hydrogen storage.
Boron Monosulfide Nanosheets
Two-dimensional Materials
Two-dimensional (2D) materials possess unique properties. They have large surface areas and new electronic states that make them ideal for many applications. These include batteries, catalysts, and electronic devices. Combining 2D materials or 2D with other-dimensional materials can result in new physical properties. Unusual properties such as superconductivity have been observed in 2D superlattices created by stacking two sheets of 2D materials and twisting them by a slight angle relative to one another.
Therefore, 2D materials can be used as building blocks to create new materials with desired and manageable functions. They also help develop new materials with desirable and controllable functionalities. Among them, boron-related substances are fascinating because of their polymorphism and their ability to form multi-center bonds.
2D Boron Monosulfide Nanosheets
Theoretically, boron can be found in a variety of 2D phases. These include borophene, hydrogenated boron (borophane/hydrogen boride), 2D boron monosulfide (2BS), 2D boron dioxide, and 2D boron phosphide.
Tuning the bonding patterns of 2D-boron nanomaterials can result in several building blocks. Experimentally, however, only borophane and boric acid have been achieved. No other 2D compounds of boron have been reported. A recent theoretical study found that 2D Boron Monosulphide can exist in several stable phases, each unique electronic structure. It includes superconducting, thermoelectric, and hydrogen storage.
Cathodoluminescence and ultraviolet-visible absorption spectroscopy and excitation-emission matrix experiment results show a constant bandgap difference between the BS nanosheets (r-BS) of about 1.0 eV. Many applications use the bandgap difference between r-BS nanosheets and BS nanosheets, demonstrable by the photoelectrochemical current switch.
Results show that changes in the nanosheet bandgap can adjust the number of stacked 2D Boron Monosulfide nanosheets. BS nanosheets, non-metal 2D materials, are promising for applications that require bandgap control, such as electronics or photocatalysis.
Tsukuba Researchers Synthesize 2D Boron Monosulfide Nanosheets
Researchers at the University of Tsukuba have just made theory into practice by synthesizing 2D boron monosulfide nanosheets. These nanosheets can be manipulated layer-by-layer to alter their electronic properties. The team managed to obtain boron monosulfide nanosheets for the first time and demonstrate unique electronic functionality that can be controlled by changing the number of layers in the stack.
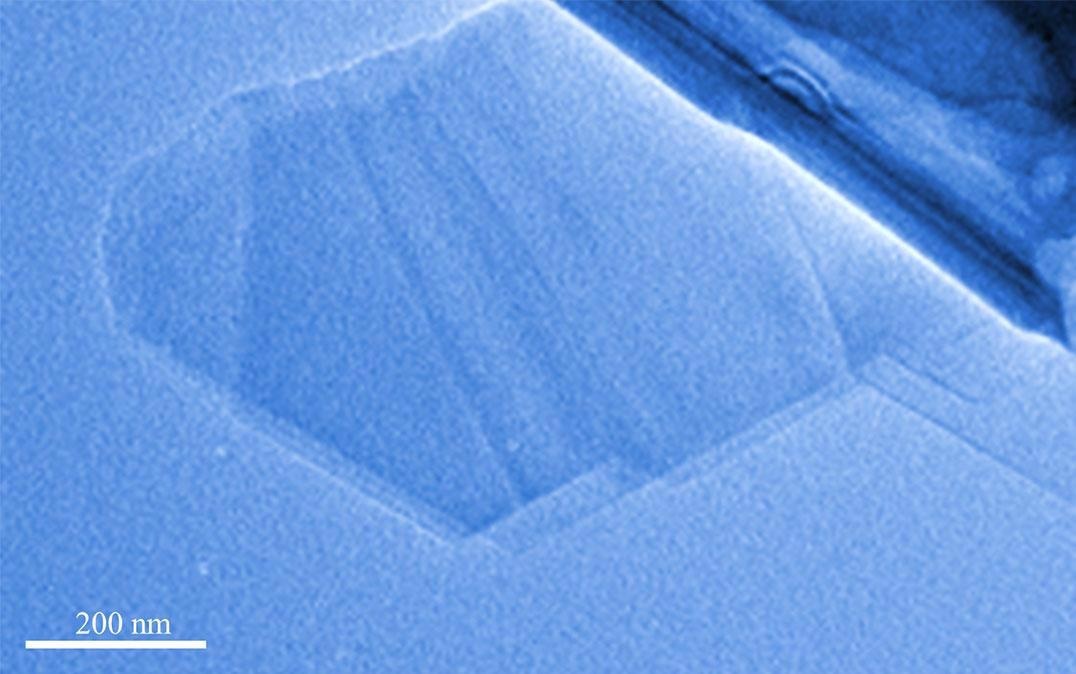
Research at University of Tsukuba into boron monosulfide nanosheets
Source: Tsukuba University https://www.azonano.com/news.aspx?newsID=38215
The physical exfoliation of bulk rhombohedral boron monosulfide (rhombohedral BS) may be used to create 2D BS nanosheets. It is possible to characterize r–BS, as it is thermally stable since only a handful of groups reported r–BS synthesis. This is checked by exfoliating r-BS and 2D BS nanosheets and can create distinctly different bandgaps. It is reproducible using density functional theory calculations (DFT). Further analyses will reveal that bandgap energy is dependent on the number of BS nanosheets stacked. These findings suggest that 2D BS nanosheets could be band gap-tunable materials suitable for advanced applications.
Boron is a non-metal multifunctional component. However, chemists only hypothesized the beneficial properties and applications of two-dimensional Boron-containing materials in the past five years.
The AZoNano report reveals that 2D materials are excellent candidates for applications in batteries and other devices due to their large surface areas and various electronic statuses. Incorporating 2D building blocks in groundbreaking materials can give greater control over their functionality.
Bandgap Tuning
After publishing their research in the Journal of Materials Chemistry, the researchers created a 1:1 boron/sulfide bulk substance. This material has a rhombohedral and specifically a 3D-rhombus crystal structure, or r–BS.
The nanolayers were removed, but the original crystal arrangement was retained. Research group leader and Professor, Takahiro Kondo, says, “Our analysis confirmed what our own calculations had predicted. That is, BS nanosheets had a different band gap energy than the bulk material, and importantly, the band gap could be tuned based on the number of stacked 2D BS sheets.“
The bandgap energy is essentially a function of the material’s ability to conduct electricity. It is, therefore, a property that helps to make potential electric devices. Researchers also found that the bandgap energies of a single BS nanosheet were quite large. However, the bandgap energy of a single BS nanosheet was still considerable. It reduced slowly as additional layers were added. After the addition of sheets (about five), the stack’s bandgap level reached bulk r-BS.
Potential Applications
The ScienceDaily similarly reports that electrodes with r-BS and 2D BS had unique bandgaps and responded to different wavelengths of light. Boron is a fascinating material. The light-driven phenomena demonstrated that 2D Boron monosulfide materials can be used in photocatalytic or electronic devices.
It is possible to modify the number of nanosheets to adjust their properties. The r-BS required lower-energy irradiation, such as visible light, to produce a current and exhibit photocatalytic activity. This research indicates that the 2D BS had a larger bandgap under higher-energy ultraviolet radiation.
Boron Nanosheets and Battery Technologies
Using nanotechnology to manufacture batteries has many benefits. One is the increased power of the battery and the shorter time it takes to recharge. Further, nanoparticles can be used to coat the electrode’s surface. This increases the electrode’s surface area and allows more current flow inside the battery.
This strategy could increase the efficiency of hybrid vehicles by significantly reducing the weight and power requirements for the batteries. Nanomaterials could also be used to improve a battery’s lifespan. Additionally, they can separate liquids from solid electrodes, thereby increasing the battery’s shelf-life. This separation stops low-level discharge from a conventional battery and dramatically increases the battery’s shelf life.
Boron Nanotechnologies for Batteries
Other Research into Boron Nanotechnologies
Parallel research into Boron nitride Nanotubes already shows different compounds of Boron can form nanomaterials that will transform energy. Boron nitride Nanotubes have potential uses in strengthening lightweight materials such as polymers and plastics. It is also being studied for its possible use in electronics, aerospace engineering, and optical fibers. Over the last few years, the market for Boron Nitride nanotubes has been steadily growing. Boron Nitride nanotubes can increase the viability and economic value of renewable energy sources like solar power.