Northwestern University Achieves a Breakthrough
Much credit is due to the team of engineers from Northwestern University who have create a double layer of Borophene for the firrst time. This runs counter to the natural tendency of Boron to form non-planar clusters beyond the single-atomic-layer limit. A single-atom layer of Borophene is much in demand in industry due to its conductuitiy and flexibility. The commericalisation of doubnel-layer Borophene will offer even better performance for such applications as energy efficiency.
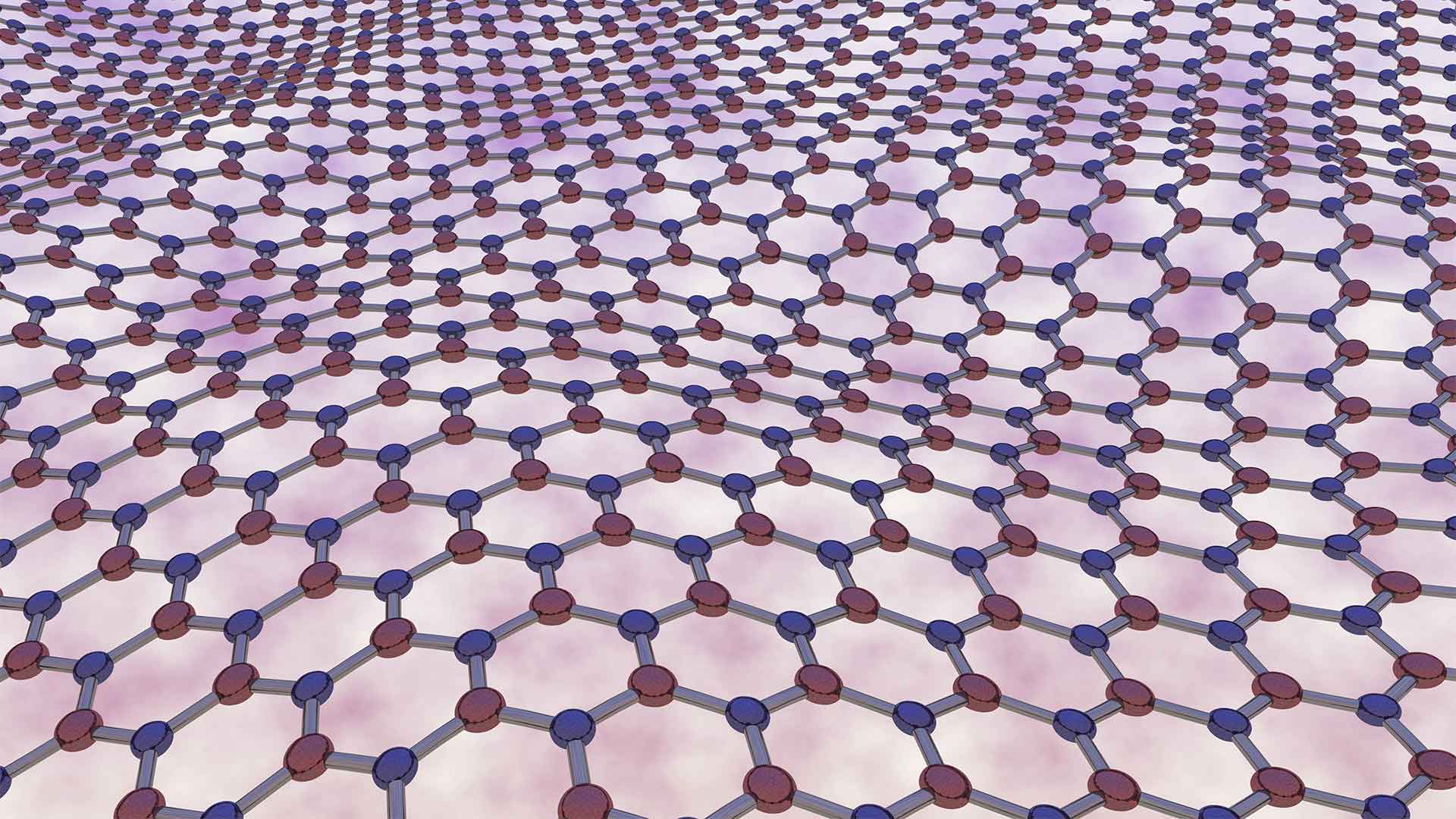
Borophene is very different from its analog counterpart Graphene. This is a two-dimensional material that is peeled from layered graphite. The peeling away process is as simple as the help of scotch tape. However, Borophene cannot be peeled away similarly from bulk Boron. It is a challenging and complicated process to create layers of Borophene.
One of the co-senior authors from Northwestern University, Mark, C Hersam says, “When you try to grow a thicker layer, the boron wants to adopt its bulk structure.”
“Rather than remaining atomically flat, thicker boron films form particles and clusters. The key was to find growth conditions that prevented the clusters from forming. Until now, we didn’t think you could go beyond one layer. Now we have moved into the unexplored territory between the single atomic layer and the bulk, resulting in a new playground for discovery,” he added.
The research article was published on August 26th, 2021.
Hersam works as the Walter P. Murphy Professor of Materials Science and Engineering at the McCormick School of Engineering and director of the Materials Research Science and Engineering Center. Additionally, he belongs to Northwestern’s International Institute for Nanotechnology and the Simpson Querrey Institute. Hersam’s co-workers in this particular research include Boris Yakobson, the Karl F. Hasselmann Chair in Engineering at Rice University.
They also create Borophene for the first time 5 years ago. It was constantly in comparison with graphene. Borophnene is lighter, stronger, and a lot more flexible than the latter. Hence, it offers a variety of applications ranging from batteries, electronics, sensors, quantum computing, and solar cells among others.
Double-layer Borophene
While a double-layer borophene sounds exciting on paper, many researchers including Hersam were not convinced of its plausibility.
The co-author feels that it is difficult to create new material. It can be hard even when theoretical work existence. He added, “Theory rarely tells you the synthetic conditions needed to achieve that new structure.”
The team confirms that the double-layered borophene offers all the existing properties of the single-atom structure. It also has many new exciting properties apart from the older ones.
Hersam and his team are now confident to create more layers from this wonder material, Borophene. They have enough inspiration to grow thicker layers or even double-layers from atoms of different geometries.
“Diamonds, graphite, graphene, and carbon nanotubes are all based on one element (carbon) with different geometries,” the team confirmed. “Boron appears to be just as rich in its possibilities, if not more so, than carbon. We believe that we are still in the early chapters of the two-dimensional boron saga.”
Hersam’s team figured out that the main key to achieving the correct conditions was depending on the substrate necessary for growing the material. They managed to raise the borophene on a silver, flat substrate.
The silver bunches form large terraces that are exceptionally flat when they exposed the material to a very high temperature. The flat surface was visible in between bunches of atomic-scale steps.
Northwestern University’s team believes that there is a promising material for batteries from bilayer borophene. They are hoping for the prediction to come true very soon.
What is so special about Borophene?
Borophene is a new form of boron with an atomically flat surface. This material is in the same family as graphene. And hence, it has attention for its potential in many applications. Borophene’s properties are very similar to graphene but it has some unique properties that make it even more interesting.
Since, Borophene is a new, highly conductive, and flexible material. It is more than twice as effective as graphene at conducting electricity and it can be made from copper and boron powder. So far, borophene is available in tens of nanometers in size but they expect it to grow into sheets just like graphene. The potential applications for borophene include use in solar cells, touch screens, and light-emitting diodes (LEDs). Additionally, you can find them in water filters, and many other devices where electrical charge needs to flow freely.
Multiple research studies are being done into Boron and Borates-derived applicaitons to help decarbonization and advanced energy initiatives as part of our efforts to save the planet for future generations.