Boronic Acid
Boronic Acid is similar to boric acid, where an alkyl or aryl group replaces one of the three hydroxyl groups. It is a compound containing a carbon-boron bond, and hence the members of this class are part of the larger class called organoboranes.
Introduction to Boronic Acid
Boronic Acid, or acids, works as Lewis acids, and they form reversible covalent complexes with sugars, amino acids, hydroxamic acids, and others. It is considered a unique feature of the acid group. They are extensively used in organic chemistry in the form of chemical building blocks. Additionally, these acids play a predominant role as intermediated in the Suzuki coupling procedures. Transmetallation is a crucial concept in its chemistry wherein the organic residue is converted to a transition metal.
The functional group of the acid features low inherent toxicity. It is one of the top reasons for boronic acid’s role in Suzuki coupling and the synthesis of pharmaceutical agents. But, boronic acids and their derivatives positively affect the Ames test, and hence, they act as chemical mutagens.
The chemical formula of Boronic Acids
Boronic Acids are compounds having the chemical structure: RB(OH)2.
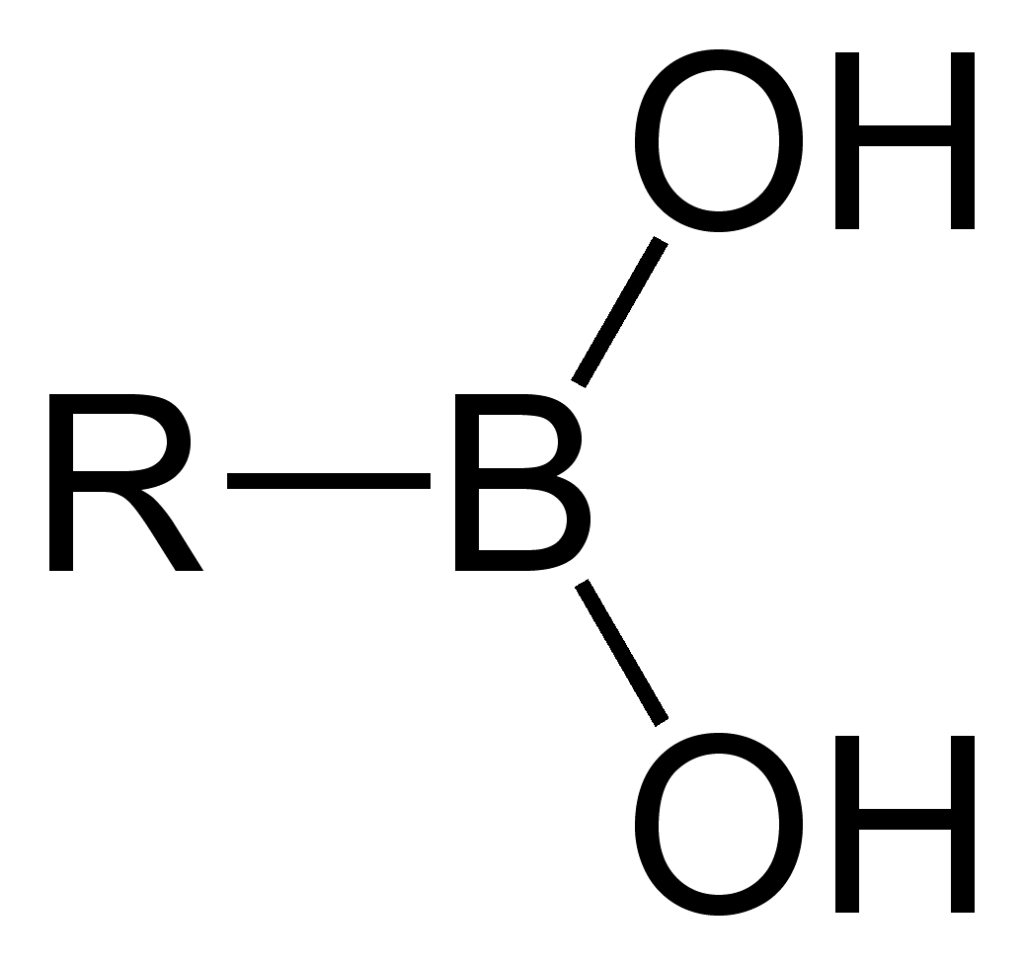
Chemical structure of Boronic Acid Source: wikipedia: https://en.wikipedia.org/wiki/Boronic_acid
They are used for molecular recognition in fluorescent detection to bind the saccharides. You can also use them for selective transportation of saccharides across membranes.
The Boronic acid group and its derivates are used in various applications, from bioorganic and medicinal chemistry to chemical biology. In the former, the acid is essential in designing and synthesizing sensors that support the detection of amino acids and carbohydrates. Then, in chemical biology, they are helpful for the detection of the tetraserine motif in protein. Additionally, they also play a crucial role in the recognition and sensing of peroxides and the MRI contrast agents development. In medicinal chemistry, boronic acids help in the preparation of inhibitors of hydrolytic enzymes. These enzymes are helpful in boron neutron capture therapy (BNCT), quorum sensing inhibition, and antifungal agent development.
Alkenyl and Alkyl Acids
When boric acids are replaced with a carbon-boron bond, then they are called alkenyl and alkyl acids. These acids are represented as R-B(OH)2. Additionally, they act as Lewis acids and are green compounds due to their low inherent toxicity properties. The alkenyl and alkyl acids expose rapid environmental degradation. These acids are commonly used for boronic acid derivative formation and other similar synthesis applications.
Aryl Boronic Acids
Aryl Boronic Acids readily undergo dehydration reactions. And most of these acids lead to a cyclic trimer anhydride. The acid and the anhydride put in similar efforts in Suzuki coupling reactions. Hence, the two of them are considered equivalent. There are different types of aryl boronic acids ranging from unsubstituted aryl boronic acids, monosubstituted aryl boronic acids, disubstituted aryl boronic acids, trisubstituted boronic acids, tetrasubstituted boronic acids, and penta-substituted aryl boronic acids.
Heteroaryl Boronic Acids
A commonly used synthetic intermediate in Suzuki Miyaura palladium-catalyzed cross-coupling reaction and others are the Heteroaryl Boronic acids. They are heterocyclic and aromatic building blocks. You can find these boronic acids in different chemical reactions such as Chan-Lam coupling, homologations, Sonogashira coupling, Stille coupling, conjugate additions, and electrophilic allyl shifts.
Boronate Esters
Boronic acids form esters with diols in an aqueous solution as a reversible reaction. It is a very significant feature of boronic acids. The Boronate esters are air-stable and chromatographically stable as well and hence are perfect for spectroscopic study.
We can use Boronate esters with the Suzuki-Miyaura cross-coupling reactions. However, there is a problem with that due to reaction scheme incompatibilities among the synthetic reagents. To avoid the problem, we can use boronic ester counterparts to counteract. Hence, they will be more compatible with many artificial schemes. But it is essential to remember that the liberation of boronic acid needs harsh conditions to interfere with the synthetic substrates.
Borylation reagents
Synthesis of boronates requires a powerful tool while cross-coupling of borylation reagents using aryl and vinyl halides. Miyaura borylation is the right tool for the process. Different chromatographic techniques help to purify the Borylated products, and they are air-stable. When the product is activated strongly, it can help to initiate the competing Suzuki coupling. Hence, it is crucial to select the suitable base for the borylation reaction to succeed.
To create C-B bonds, we usually use lithium or Grignard reagents as a combination with an electrophilic source of boron. However, some of the functional groups are not tolerated properly. It is due to the nucleophilic and the essential nature of the metal species in the two-step procedure. The very mild conditions from the borylation reaction give space for the preparation of boronates. It is not otherwise accessible through lithium or Grignard intermediates.
MIDA Boronates
A class of caged boronic acids is called MIDA boronates. They work extraordinarily in Suzuki-Miyaura cross-couplings iterations. The MIDA boronates are also very robust when they contact the common harsh reagents like Jones Reagent. They can transform the derivative without any changes to the MIDA component.
How to make boronic acids?
Edward Frankland reported the preparation and isolation of boronic acid for the very first time in 1860. He used a two-stage process to synthesize Ethylboronic acid. Initially, the reaction between diethylzinc and triethyl borate produced triethylborane. Then, this compound was oxidized in the air to create ethyl boronic acid. However, today there are several synthetic approaches and methods to produce the acids. Additionally, different boronic acids are available commercially, and they are air-stable too.
The boronic acids showcase high melting points. They quickly form anhydrides just with the loss of water molecules, paving the way to develop cyclic trimers. Apart from the straightforward methods, there are other approaches to produce Boronic acids as well.
Methods of preparation
One of the standard methods is the reaction of organometallic compounds. They are based on lithium or magnesium Grignards with borate esters. Consider an example where phenylmagnesium bromide reacts with trimethyl borate to produce phenylboronic acid. Later, the process is followed by hydrolysis.
We have yet another technique with the concept of transmetallation. Here, aryl silane reacts with boron tribromide and undergoes a transmetallation to RBBr2, followed by acidic hydrolysis.
Finally, we have a coupling reaction called the Miyaura borylation reaction. In this method, aryl halides and triflates react with diboronyl esters under a palladium catalyst. We can also use diboronic acid or tetrahydoxydiboron as an alternative to esters in this method.





