Boron Neutron Capture Therapy
Every day, oral cancer is on the rise. Radiotherapy, chemotherapy, and surgery are the main treatment options for cancer. Although surgical annihilation works well in primary tumours, it is ineffective for smaller and more accessible tumours. As a result, cancer cells may not be entirely eradicated by this method. Chemotherapy uses chemical drugs to combat cancer. Boron Neutron Capture Therapy may play a part in disease reduction.
Boron Neutron Capture Therapy
The body circulates the systemically administered drugs to kill rapidly growing cancer cells. Due to its toxic effects on normal cells can cause serious side effects and may lead to resistance in cancer cells.
Radiation uses high-energy ionization particles such as X-rays and gamma rays to cause damage to cells at the molecular level. It is commonly used to kill remaining cancer cells following surgery. It can also damage healthy tissues and the surrounding cancer cells.
How Boron Neutron Capture Therapy Works
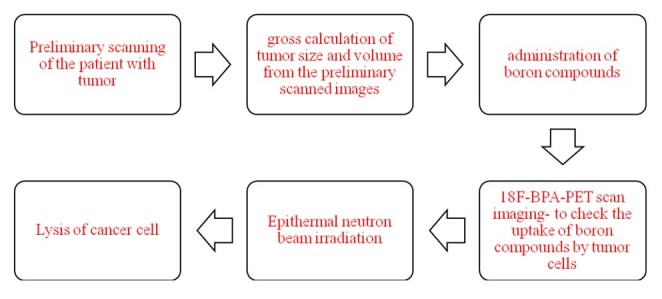
Boron Neutron Capture Therapy (BNCT) is a treatment that uses Boron to target cancer cells. It works by concentrating boron in a tumour and then exposing it to thermal neutrons.
BNCT Neutrons are delivered at a high rate of flow and with sufficient energy. Radiation beams directed into the tumor should not contain any contaminants. The nuclear reactors and accelerator-based neutron sources (ABNS) are the source of the neutrons for epithermal radiation. The majority of these nuclear reactors have been closed, are on the verge, or have stopped their BNCT trials activities.
Here is how Boron Neutron Therapy works:
Boron Neutron Capture Therapy
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5296588/figure/F3/
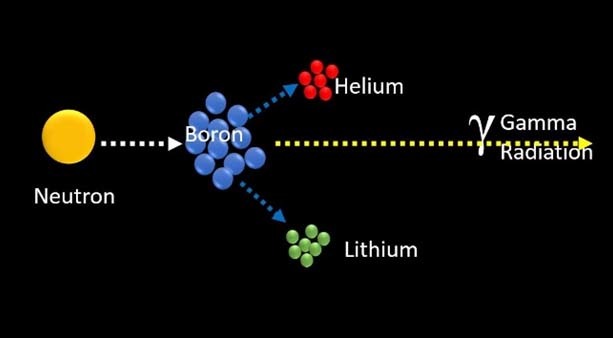
The 10B isotope, a non-radioactive isotope, absorbs low-energy (0.5eV), thermal neutrons. It then breaks down into a Helium-4 particle, and a recoiled Li nucleus (7L). The result is the high Linear Energy Transfer (LET) alpha particle at 150 keV/mm and 7Li ion at 175 keV/mm.
Boron Neutron Capture Therapy
10B + Ni—-> 7Li + 4He
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5296588/figure/F3/
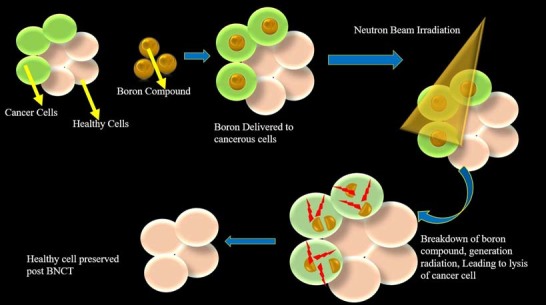
These particles offer high energy along a concise pathway (10mm). Because they are so small, their energy deposition can only be limited to the dimensions of one single cell. Only neoplastic with 10B is affected by thermal neutron irradiation. Hypothetically speaking, any normal cells adjacent to the cancer cells will be spared from high LET irradiation with 4 He and 7 Li particles.
Boron Neutron Capture Therapy 3
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5296588/figure/F3/
The radiation doses delivered to tumours and other normal tissues by BNCT are due to energy deposition. Three types of directly ionizing radio differ in their linear energies transfer (LET). This refers to the rate at which energy is lost along the path of ionizing particles. High-LET, heavier-charged alpha particles (stripped helium nuclei [4He]) and lithium-7, which are the products of thermal neutron capture and fission with 10B (n, a),7Li).
Because both the tumor and surrounding normal tissues are in the radiation field, even an ideal epithermal nucleon beam, there will inevitably be a background dose that consists of both high-LET radiation and low-LET radiation. A tumour with a higher 10B concentration will receive more radiation than normal tissue. This is what gives rise to the therapeutic benefits of BNCT.
Why Boron is Used for Neutron Capture Therapy
Boron neutron capture therapy (BNCT) can be used to treat malignant tumours. It is non-invasive and painless. It uses neutrons to produce energetic alpha particles, killing most cancer cells but not the surrounding tissues.
Tumour cells must contain enough of the boron to enable their lysis. It has been shown that using tumour-targeting moiety combined with nanoscale drug distribution via liposomes, nanoparticles, and nanoparticles is the most effective way to kill tumour cells.
The BNCT has been clinically evaluated as an alternative to radiation therapy for malignant brain tumours like glioblastomas.
Top Properties of Boron compounds High systemic toxicity, normal tissue and high uptake of tumours (>3-4%), tumour concentrations 20-30mg10B/g tumor, rapid clearance from the blood, normal tissues, and persistence in tumors during BNCT
The boron compound is currently available in three versions. Each generation has undergone continuous refinement.
- A) 1st-generation Boron Compounds Boric acids derivatives were used as clinical compounds in clinical trials.
- B) 2nd generation Boron Chemicals: BPA, a boron-based compound, was discovered in 1960. BSH, BPA, and other related compounds were also readily available. Their tumor/brain/blood ratios are higher than other similar compounds, and they are being used in clinical trials and research.
- c) 3rd Generation Boron Complexes This is a stable or cluster of boron that has been linked via a hydrolytically stabilized linkage with a cancer-targeting component or moiety. (Boron Carriers). These compounds have been used to target tumors by using low- and high-molecular-weight biomolecules. These three-generation compounds tend to be more effective in targeting the tumor cells. The target cell nucleus and DNA can be attractive targets. Additionally, toxic boron can cause death in a small area.
A recent technological breakthrough in compact accelerator-based production of neutrons is another reason BNCT has been gaining renewed interest. Because of the current technological breakthrough in close accelerator-based production of neutrons, BNCT is now possible in hospitals.
Principles of Boron Neutron Capture Therapy
Boron neutron captured cancer therapy (BNCT), a therapy for cancer that uses neutrons to capture 10B (n.a) 7Li, is a neutron capture treatment. The reaction of 93.7% of He to He (alpha particle) with energies of 1.47 MeV, Li at 0.48 MeV gamma, and 0.48 MeV energy, respectively. The rest is the decay (6.3%) of lithium, which produces alpha and Li, with respective energies of 1.78 MeV. BNCT uses the ionization energies of alpha and lithium to disrupt the structure of DNA, RNA, and other cancer cells.
The actual particles can kill cancer because they contain helium nuclei which are significantly heavier than sub-atomic particles. Particle ionization proved to be an efficient agent. It has a high level of linear energy transfer (LET), in the range 100 kV / mm, with very high efficiency for keeping on range short tissue (50–100) mm. First, boron 10 (also known as BSH, BPA) was sent to a cancer cell by concentrating 20 mg/g or 109 electrons/cell on BNCT.
After this, cancer cells contain boron ten at radiation with thermal neurons with energy 0,025eV. A reaction 10 B (n.a.) 7 Li will take place on cancer cells that have boron. Lithium and Alpha 7 are used to ionize cancer cells. Both the alpha particle and the BNCT result from lithium react to produce transfer energy linear.





