Is Boric Acid Or Borax Effective For Home Pest Control?
Boric acid and borax have been in use as home remedies for controlling pests like cockroaches and ants for many years. But are they effective? And are they safe to use around the home?

Boric Acid
What is Borax?
Borax is a boron compound found in a white, powdery form. It is a commonly used mineral as it dissolves easily in water.
Borax has a long history of use in the United States and worldwide. It was even used as a flux in gold mining to obtain the metal without mercury. Today, borax is used for its many benefits, including being a fire-retardant, a laundry detergent, a water-softening agent, a preservative, and an antifungal.
Borax is a naturally-occurring mineral first reported to be used in Tibet in the 700s from dried-up lake beds. It was traded along the Silk Road and didn’t become widely available till the late 1800s.
Molecular Structure of Borax
Borax is a sodium borate compound that refers to sodium borate in its hydrated form, having 10 water molecules for every sodium borate entity. There are two kinds of boron atoms in this compound’s chemical structure: two out of the four boron atoms shape four coordinate bonds, while another two boron atoms shape three coordinate bonds and have tetrahedral and triangular structures, respectively.
What is Boric Acid?
Boric acid is a compound with a wide range of applications, from being used as an insecticide to serving as a flame retardant. It is a white powder or colorless crystal that dissolves easily in water and has a slightly acidic taste. Although it is classified as a weak acid, it can be corrosive and irritate if it comes into contact with mucous membranes or broken skin. In its mineral form, it is known as sassolite and can be found in volcanic areas. Boric acid is also a component of other minerals, such as borax, boracite, and colemanite.
Wilhelm Homberg first prepared this compound in 1702. He had combined borax and mineral acids. On the other hand, borates were used during the time of the Greeks.
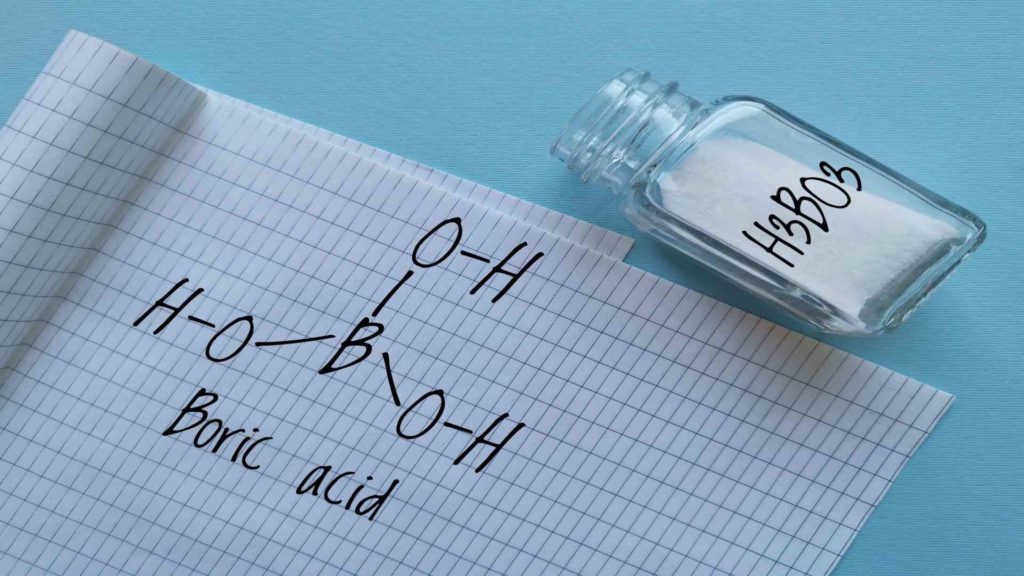
Molecular Structure of Boric Acid
Boric acid has a trigonal planar arrangement of boron atoms. Boron can only form three covalent bonds because it has three unpaired electrons. In boric acid, these bonds are formed with the –OH groups surrounding the boron atoms.
This acid is a lewis acid as incoming electrons from donor molecules can fill vacant orbitals on the boron atom. Any molecule or ionic species that can accept a pair of electrons from another molecule is referred to as a Lewis acid.
When the compound reacts with water, it forms B[[OH4]]- due to the binding of a hydroxyl (–OH) group from one water molecule, which donates its electron pair to the boron’s empty orbital. Orthoboric acid is also categorized as a monobasic acid, which means it has only one hydrogen atom to be replaced.
Borax vs Boric Acid
| Properties | Borax | Boric Acid |
|---|---|---|
| Chemical Formula | Na2B4O7·10H2O | BH3O3 |
| Other Names | sodium borate, sodium tetraborate, disodium tetraborate | Hydrogen borate, Boracic acid, and Orthoboric acid |
| IUPAC Name | Sodium tetraborate decahydrate | Trihydrooxidoboron |
| Crystal Structure | Monoclinic | Trigonal Planar |
| Compound Nature | Salt | Weak, monobasic Lewis acid |
| Molecular Weight | 381.37g/mol (hydrated), 201.22 g/mol (dehydrated) | 61.83g/mol |
| Appearance | White crystalline solid | White crystalline solid |
| pH Value | 9.5 | 5.1 |
| Melting Point | 743°C | 170.9 °C |
| Boiling Point | 1,575°C | 300 °C |
| Density | 1.73 g/cm3 | 1.435 g/cm3 |
| Solubility In Water | 31.7 g/L | 2.52 g/100 mL (0 °C) 4.72 g/100 mL (20 °C) 27.53 g/100 mL (100 °C) |
Which is Recommended? Borax Or Boric Acid?
Boric acid and borax are both similar in many ways. They are both boron compounds, and they can both be used for cleaning products. However, boric acid is more refined and processed than borax. It is also essential to take extreme caution when using either of these products around children and pets, as they can be toxic if ingested.
Boric acid is one of the most effective insecticides that comes in tablet, liquid, powder, or trap form. It poisons insects’ stomachs, disrupts their metabolism, and removes their exoskeletons. Its smaller grains make it harder for pests to detect and easier to ingest, making it more effective at killing pests than borax. It sticks to insects when they come into contact with it. They then ingest the acid while cleaning themselves, which ultimately leads to their death.
Because high concentrations of the compound can repel insects, most baits containing it only contain a 5% formulation. On the other hand, fine powders or dust contain 98-99% orthoboric acid once spread out in a thin layer.
What Kind of Pests does Boric Acid (Orthoboric Acid) Kill?
Orthoboric acid is commonly touted as a panacea for all sorts of household pests, but the truth is that it only works on certain types of insects. To be effective, the bug must ingest the acid after grooming itself – meaning that creatures who don’t clean themselves regularly, like spiders, are immune to its effects.
The most common pests that can be killed with this compound are ants and cockroaches.
What Kind of Pests Orthoboric Acid does not Kill?
Unfortunately, orthoboric acid does not kill all types of pests. Thus, it is best to contact a professional pest control company with any of the following pest problems:
- Ticks
- Bugs in beds
- Flies
- Moths
- Beetles
- Centipedes
- Spiders
- Hornets and Wasps
Boric acid is useful for pest control but is also toxic to humans if consumed. As a result, while using at home, it is advised to exercise extreme caution. It is best to avoid using it near food or children’s toys and to clean up any residue as soon as possible.
Other Uses of Orthoboric Acid
Boric acid is an inorganic compound with numerous applications. It was initially meant for medical use. However, since the industrial revolution, it has been used for a variety of other benefits, including,
- treating yeast infections,
- as an antiseptic for minor wounds,
- as a fire retardant,
- cleaning contact lenses,
- removing stains from the fabric,
- and as fireproofing agent for wood, etc.