Alkaline Balancing pH is a key component to creating an environment that is healthy for life. Boron, a non-metal found harmlessly and naturally combined with oxygen as borates, helps create this balance. Boron is a key element that can be beneficial for your health as it proves to be essential for many bodily functions including bone development, energy metabolism, and cell signaling.
Studies show that it can help with balancing out acidity and alkalinity in the body by being used as a way to neutralize acids. In the home and in the environment, it is also an excellent sanitiser for a germ-free home found as a component of household cleaners, soaps, detergents, and laundry products.
Facts About Boron
- Boron is an inert element, so it does not react with all acids and alkalies. Its acidity can help balance alkalinity. When considering how much borate there is in solutions, more ionization will take place if there are higher concentrations of alkaline materials present.
- Boron reacts with sodium hydroxide to form sodium borate which dissolves into alkalies. However, some of its behavior towards some chemical reactions suggest it to be tribasic acid in the Brønsted -Lowry acid-base theory.
- When the pH of water is lower, it contains more hydroxide and boron. When alkalinity increases, so do the amount of borate anion and vice versa.
- Boron is a non-metal which means that it accepts electrons, while acids donate electrons, so it should react in acids but it doesn’t.
- There is no preference in reactivity towards acids or bases either.
- Boron indeed doesn’t appreciably react either with non-oxidizing acids or with alkali solutions under ambient conditions. The only exception is hydrochloric acid with boron.
- The amount of borate anion is greater in more alkaline (or higher pH) solutions. This means that the solution can be made less acidic by adding a substance, such as sodium hydroxide or potassium carbonate to it.
- Well, boron can be used to help in balancing acidity and alkaline by being able to form compounds with more acidic elements such as nitrogen in nitric oxide (NO) or sulfuric acid (HOSO).
- We may call it “basic” by virtue of the products being much weaker acids than nitric acid.
- When the water pH changes, boron is either given off or absorbed. The change in levels of borates reflects this balance between acids and alkalines.
COMPOUNDS OF BORON
BORAX
Boron Crystals
Borax is obtained as a mineral and it can be found in nature that can be found combined with oxygen or other elements, it’s one of the most well-known borates. It dissolves in water to give an alkaline solution. It reacts with sodium hydroxide to form sodium borate which then dissolves into alkalies and releases hydrogen. Borax dissolves in water to give an alkaline solution. Na2B4O7 + 7H2O → 2NaOH + 4H3BO3 Orthoboric acid
Also, borax is obtained by acidifying (dissolving boric acid) the aqueous solution of it with hydrochloric acid which then evolves hydrogen gas as a result of the interaction. Borax is an alkaline material that can be used for pH balancing without raising or lowering your acid concentration.
ORTHOBORIC ACID

Source: pureborax.com
Orthoboric acid is prepared by acidifying the aqueous solution of borax with hydrochloric acid. Boracic acid and orthoboric acid are weak monobasic Lewis acids of boron. It is not a protonic acid but acts as a Lewis acid by accepting electrons from a hydroxyl ion: B(OH)3 + 2HOH → [B(OH)4] – + H3O+
On heating, orthoboric acid above 370K forms metaboric acid, HBO2 which on further heating yields boric oxide, B2O3. H3BO3 ⎯Δ → HBO2 ⎯Δ → B2O3
DIBORANE
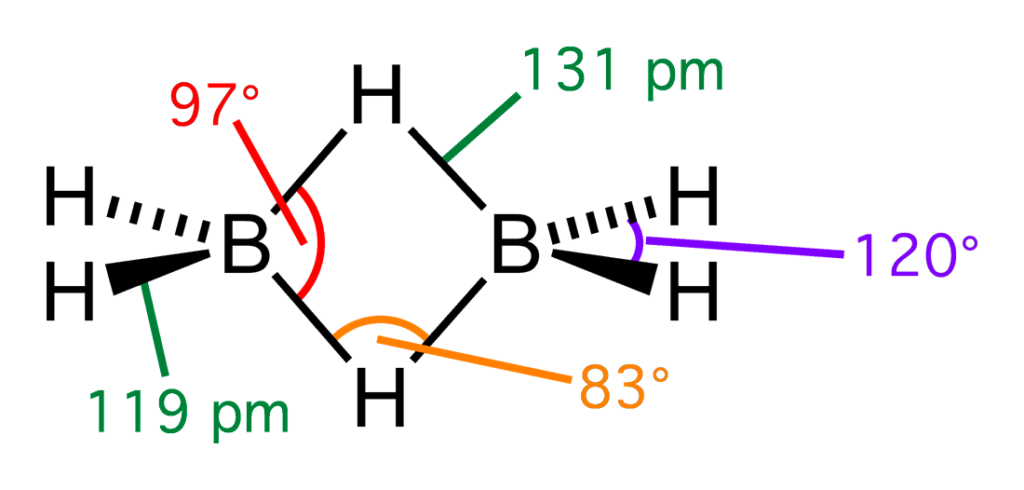
Source: https://en.wikipedia.org/wiki/Diborane
DIBORANE is the simplest form of boron hydride which is a colorless, highly inflammable gas with an unpleasant odor. Diborane catches fire spontaneously upon exposure to air. It burns in oxygen releasing an enormous amount of energy. Most of the higher boranes are also spontaneously inflammable in the air. Boranes are readily hydrolyzed by water to give boric acid.
B2H6(g) + 6H2O(l) → 2B(OH)3(aq) + 6H2(g)
Diborane undergoes cleavage reactions with Lewis bases(L) to give borane adducts, BH3⋅L B2H6 + 2 NMe3 → 2BH3⋅NMe3 B2H6 + 2 CO → 2BH3⋅CO
BORON IN BALANCING AND ITS USES
In Industry
- Boron being extremely hard, refractory solid of high melting point, low density, and very low electrical conductivity find many applications in the industrial sector due to its properties like corrosion resistance, hardness, etc.
- The amount of borate anion is greater in more alkaline (or higher pH) solutions as it exists predominantly as a univalent ion at these.
- As a coating for jet engine blades to protect them from corrosion. Boron compounds are also used in the production of nuclear weapons and other military applications, e.g., armor-piercing shells and rifle bullets.
- Boron fibers are used in making bullet-proof vest as it is difficult to penetrate. It also finds application in the production of high-performance ceramics and can be found in a few different industrial lubricants such as Lithium Borohydride (LiBH)
- the boron-10 (10B) isotope has a high ability to absorb neutrons and, therefore, metal borides are used in the nuclear industry as protective shields and control rods.
- The main industrial application of borax and boric acid is in the manufacture of heat-resistant glasses (e.g., Pyrex), glass-wool and fiberglass as they form borosilicate glass and are used as fluxing agents in the production of high purity metals.
In Nature
- Boron is a natural element that can be found in the air we breathe and control acidity levels.
- In nature, boron compounds are almost exclusively found as minerals or salts formed by water that has interacted with various mosses, algae, fungi, and bacteria growing on the earth’s surface.
- Borax is a compound used to control the pH of the water and so helps in controlling diseases such as malaria by fighting protozoan parasites that cause it.
- In nature, it is found combined with oxygen and other natural elements forming several different compounds called borates.
In Households
- Borax has many uses: it can be used as a cleaning agent for bathroom surfaces because of its natural antibacterial properties; mixed with salt to make a liquid chlorine bleach and light composite material for aircraft.
- Boron forms an excellent component of household cleaners, soaps, detergents, and laundry products.
In Humans
- Boron is also an essential constituent for plants, animals, and humans. The element is found in trace quantities (0.0018- 0.05% by weight) in plant tissues with higher concentration levels occurring more often near surfaces which can balance the acidity and basicity in all these organisms by functioning as a buffer. A buffer solution is a solution that resists changes in pH or keeps the concentration of hydrogen ions at a constant level.
- The notion of boron being “essential” for animals and humans is controversial, however, the consumption of foods high in dibasic acid has been associated with improved bone health in elderly populations leading to the healthy functioning of their bodies because it is used for the production of collagen and mucopolysaccharides.
- Boron acts as a buffer in the human organism, and as a result, it is considered to be an essential nutrient.
- An alkaline diet rich in fruit and vegetables has been shown to reduce the risk of osteoporosis, diabetes mellitus type II, cancer including prostate cancer, and Alzheimer’s disease.
- Borate ions have been found in freshwater environments such as lakes where they help in making the water supply safe by balancing the pH.
- Boron also has beneficial effects on human health such as:-
-preventing the growth of cancer cells, and stimulating bone-building; -reducing inflammation that is associated with arthritis pain relief;
– Along with other key nutrients like magnesium and calcium, boron affects hormone levels that are necessary for healthy bones:
-decreasing seizures in epileptics by altering neurotransmitter levels within the brain; it can be seen as a buffer against the effects of sodium and calcium on nerve cells;
-connecting brain function with mood, sleep patterns, learning ability, and mental performance. See more here: https://www.rxlist.com/consumer_boron/drugs-condition.htm
- What’s interesting is that boron is found in certain plants such as almonds, avocados, bananas, broccoli, cabbage family vegetables (cauliflower), garlic; and in some fruits as well (apples, pears).
In Soil – Agriculture
- The amount of borate anion in the soil helps in the balance between acidity and alkalinity. hence, proving the role of boron in the soil, indirectly, in agriculture.
- The chemical compound boron is used to make a range of products we use every day – from fertilizers for the soil to medicines and pesticides.
- Availability/solubility of boron is pH-dependent, boron deficiency is a problem on alkaline soils as boron compounds precipitate onto the soil, thus reducing availability.
- Boron is easily leached from the soil and is the most commonly deficient micronutrient in soils.