Understanding the Chemistry of Boron
Boron has interesting chemistry with varying properties, applications and isotopes. It is a semimetal that belongs to group 13 of the periodic table and is essential to plant growth and wide industrial uses.
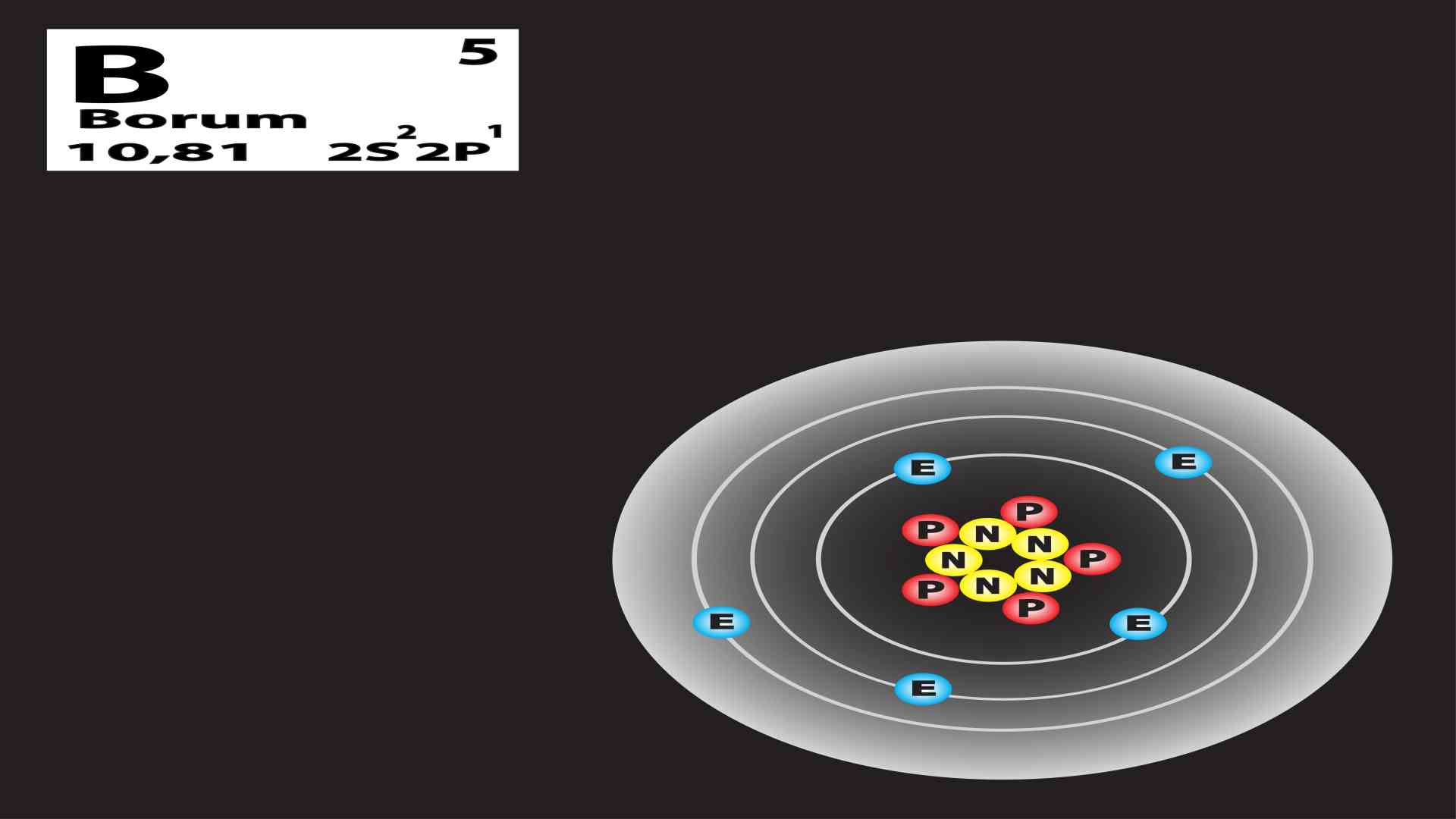
Chemistry of Boron
Boron – A Magical Element
Boron is a quirky little element with symbol B and atomic number 5. Boron originated from the Arabic and Persian words for borax, which is its principal ore. Boron is an electron-deficient element with an empty p-orbital.
It has several forms, but the most common is Amorphous boron, a dark powder that is unreactive to oxygen, water, acids, and alkalis. Also, boron is the only non-metallic element in Group 13. It possesses qualities that are between metals and non-metals (semimetals).
Boron is found in trace amounts in the earth’s crust, about 10 parts per million (about the same abundance as lead). The pure element is shiny and dark in colour. It is incredibly hard, almost as hard as a diamond in its purest form, but far too brittle for practical use. It is an excellent conductor at high temperatures, but an insulator at room temperature and below.
History of Boron
Although boron compounds was known in ancient times, Gay-Lussac and Thénard were the first to isolate boron in 1808, followed by Sir Humphry Davy (with a long list of elements to his credit).
Davy observed that an electric current passed through a borate solution generated a brown residue on one of the electrodes. Instead of electrolysis, he utilized potassium to reduce boric acid in future trials. He created enough boron to demonstrate the existence of a new element, which he termed boracium.
At high temperatures, Gay-Lussac and Thénard used iron to reduce boric acid. They demonstrated that boric acid is the oxidation product of boron by oxidizing it with air. It was recognized as an element in 1824 by Jöns Jacob Berzelius. Ezekiel Weintraub, an American scientist, is credited with being the first to create pure boron in 1909.
Preparation of Boron in the Laboratory
Reducing boric oxide with metals like magnesium or aluminium was one of the early methods of obtaining elemental boron. The borides of those metals, however, are always present in the product.
Volatile boron halides can be reduced with hydrogen at high temperatures to produce pure boron. The breakdown of diborane at high temperatures has ultrapure boron for use in the semiconductor industry, which is then refined further using the zone melting or Czochralski processes.
The synthesis of boron compounds does not need the formation of elemental boron; instead, it takes advantage of the readily available borates.
Facts about Boron
| Boron properties | Details |
|---|---|
| Group | 13 |
| Period | 2 |
| Block | p |
| Atomic Number | 5 |
| State at 20°C | Solid |
| Electronic Configuration | [He] 2s2 2p1 |
| Atomic Mass | 10.81 g/mol |
| Stable Isotopes | 10B, 11B |
| Melting Point | 2350 K |
| Boiling Point | 4200 K |
| Density | 2.34 g/cm3 |
| Heat of Fusion | 50.2 kJ/mol |
| Heat of Vaporization | 480 kJ/mol |
| Specific Heat Capacity | 11.087 J/mol K |
| Oxidation States | 4, +3, +2, +1 |
| Magnetic Ordering | Diamagnetic |
| Electronegativity | 2.04 |
| Atomic Radius | 90 pm |
Unique Properties of Boron
- Boron and its compounds emit a green flame when ignited.
- The isotope boron-10 has a high propensity for absorbing thermal neutrons.
- The coefficient of linear thermal expansion of borosilicate glasses (SiO2 80% + B2O3 13%) is extremely low (~ 3.3x 10-6 cm/cm/ºC). They are more resistant to thermal shock than other glasses, such as soda glass (SiO2 73% + Na2O 14 ~ 8.9 x 10-6cm/cm/ ºC).
- The trivalent electron-deficient boron compounds are excellent lewis acids.
- Boron halides do not dimerize due to boron’s smaller size and higher electro-negativity.
- Boron compounds aren’t toxic, making them attractive compounds in the pharmaceutical and cosmetic industries.
- Sodium perborate, Na2H4B2O8, otherwise [B2O4 (OH)4] 2- releases oxygen at 60 degrees Celsius. This salt produces H2O2 when it is hydrolyzed. As a result of this property, it acts as an oxidant and chlorine-free bleach that is less aggressive.
- Aryl boronic acids are effective reagents for cross-coupling reactions catalyzed by palladium (Suzuki Coupling).
- Carboranes have higher air stability, forming useful and stable organo derivatives with a high boron content and steric bulkiness, resulting in weak anionic counterions.
- Boron carbide and boron nitride have hardness comparable to diamond and high melting points, making them valuable materials in the nuclear (control rods) and defence industries.
Organoboron Chemistry
Organoboron compounds are known in a large number, and many of them are important in organic synthesis. Several are produced using hydroboration, which utilizes diborane, B2H6, a simple borane chemical.
The organoboron compounds are generally tetrahedral or trigonal planar, such as tetraphenylborate, [B(C6H5)4], triphenylborane, B(C6H5)3. When multiple boron atoms react, they form novel dodecahedral (12-sided) and icosahedral (20-sided) structures composed mainly of boron atoms or carbon heteroatoms.
Organoboron chemicals have been used in various applications, including boron carbide (a complex, very hard ceramic made up of boron-carbon cluster anions and cations) and carboranes (carbon-boron cluster chemistry compounds that are halogenated to form reactive structures like carborane acid).
Carboranes are valuable molecular moieties that add significant amounts of boron to other biochemicals to produce boron-containing compounds for cancer treatment using boron neutron capture therapy.
Isotopes of Boron
Boron exist in two stable isotopes: 11B (80.1%) and 10B (19.9%). The mass difference results in a wide range of 11B values in natural waters ranging from 16 to +59, characterized as a fractional difference between the 11B and 10B and traditionally expressed in parts per thousand.
Boron has 13 known isotopes, the shortest of which is 7B, which decays via proton emission and alpha disintegrate. Its half-life is 3.5×10^-22 seconds. The exchange processes of the boron species B(OH)3 and [B(OH)4] determine the isotopic fractionation of boron.
Additionally, Boron isotopes can be fractionated during mineral crystallization, H2O phase changes in hydrothermal systems, and hydrothermal alteration of rock.
The unusual 17B exhibits a nuclear halo, which means its radius is far bigger than the liquid drop model suggests.
The isotope 10B is used for capturing thermal neutrons. Nuclear power plants enrich natural boron to nearly pure 10B. Depleted boron, a less valuable byproduct, is almost pure 11B.
Applications
Almost all the boron ore mined on the planet is refined into boric acid and sodium tetraborate pentahydrate. Boron is used for making glass and ceramics in the United States, accounting for 70% of the total.
The main industrial application of boron compounds (about 46%) is in the production of glass fibre for boron-containing insulating and structural fiberglasses, especially in Asia. Glass fibres are enhanced with boron, either as borax pentahydrate or boron oxide to improve their strength or flexibility.
Boron is also used in high strength glassware, accounting for another 10% of global production. More than 15% of global boron is used in ceramics, including extremely hard materials. Agriculture uses 11% of global boron production, whereas bleaches and detergents use roughly 6%.





