Boron Suzuki Coupling
Boron chemistry has become one of the most versatile and valuable fields in organic catalysis and synthesis. Numerous useful reactions like hydroborylations or Suzuki-Miyaura cross-couplings, suzuki coupling, are now indispensable to the synthetic toolbox of both academia and industry researchers. The development of novel C(sp3)-Boron reagents and their subsequent metal-catalyzed cross-couplings attracts strong attention and serves to accelerate the wheel of innovative applications of otherwise challenging organic adducts across multiple areas.
Both classical and new C(sp3)–B reagents have been widely used in recent years, such as trifluoroboronates and alkylboranes), MIDA, and alkyl boronic acid, MIDA, etc. Their role as coupling partners in tricky metal-catalyzed C (SP3)-C (SP2) cross-coupling reactions such as the B-alkyl-SMCs after 2001.
Boron is a rare metalloid with remarkable chemical complexity. Boron’s unusual properties stem from the three valence electrons. These electrons can easily be torn away, making them metallic and electron-deficient. However, they are sufficiently localized and tightly bound with the nucleus to allow for the formation of insulating states. Because of their low atomic mass (10.811 +-0.007 AMU), boron compounds have been extensively investigated for energy storage.
 Boron Suzuki Coupling
Boron Suzuki Coupling
Organoboron Compounds In Pharmaceuticals
Over the past two decades, several boron-containing drugs have been approved for clinical use. More are in clinical trials. Boron-containing compounds are gaining popularity due to their unique binding capabilities to biological targets. For example, boron substitution could be used to modify biological activity.
Organoboron compounds, such as boronic acid, boronic esters, and boronamides generally contain at least one carbon-boron (CB) bond. Sixty years ago, organoboron compounds were first used in organic synthesizing. These reagents have been a part of organic synthesis and catalysis since the 1960s. They are currently involved in many essential and classic reactions, including hydroboration and Suzuki Miyaura cross-couplings. SMC reactions generally involve the combination of an organoboron agent and an organic halide/pseudohalide in the presence of palladium (or another relevant metal/ligand) as a catalyst or base for activating the Boron compound.
Many organoboron compounds are also used in pharmaceuticals. Boron-based drugs represent a new class of molecules that can be used for various biomedical applications, such as molecular imaging agents (optical/nuclear imaging), neutron capture therapy agents, and therapeutic agents (anticancer, antiviral, etc.). The utility and widespread use of boron-based substances have also aided in developing agricultural sciences and material sciences.
Suzuki–Miyaura Cross-coupling (SMC) With Organoboron Compounds
SMC – suzuki coupling – refers to the combination of an organoboron catalyst with an organic halide/ pseudohalide in the presence of palladium (or another relevant metal) as a catalyst or a base for activation of the Boron compound. Because palladium is so efficient, catalysis has seen rapid advances. SMC coupling reactions are performed at ppb (parts-per-billion) molar catalyst loads. Even though nickel is expensive, it has high catalytic activity for SMC. Nickel’s high reactivity was demonstrated with challenging substrates like aryl chlorides/mesylates.
These coupling reactions are not as easy with traditional Pd catalysis. Nickel catalysts are more cost-effective than other metal catalytic systems. They can also be removed from reaction mixtures faster, economically feasible. In SMC reactions, other metal catalytic systems such as Fe, Co and Ru, Cu, Cu, Ag, and so on have been explored. Their applications are far more limited than those of Pd or Ni catalysts.
The Suzuki-Miyaura reaction was discovered in 1979 and has been one of the most popular, simple, and versatile transition metal-catalyzed methods for C-C bond construction. The catalytic cycle works similarly to other metal-catalyzed crossing-couplings. It starts with an oxidative addition and ends with a transmetalation. The Suzuki-Miyaura coupling is distinguished from other transition-metal cross-couplings by the activation or transmetalation of the boron agent.
Mechanistic studies were able to show the roles of each reagent within the reaction medium, in addition to that of the metal. Some of these insights have been established, such as the necessity for sigma-rich electron donor ligands, protic sols, and the base. Other mechanistic insights remain active research areas, including boron activation when the base is present.
Boronic Acids Derivatives for Coupling
Boronic acid, which is stable and non-toxic, is easy to synthesize. It can also be used in many synthetic reactions, including acid catalysis, Suzuki-Miyaura, acid catalysis, and hydroboration. Compounds containing organic boron are widely used as intermediate building blocks in organic chemistry. Cross-coupling metal-catalyzed Suzuki Miyaura is one of the most prominent uses of these compounds. Here, they are used to form new CC bonds. This complex reaction involves several steps, including ligand substitution and transmetalation.
Boronic acids are mainly found in the form of aryl boronic acids when they are used in drugs. Medicinal Chemistry also uses heterocycles containing boronic such as pyrrolyl, pyridinyl, and indolyl derivatives. The syntheses of these heterocycles are done similarly to aryl boronic. In cross-coupling reactions with organoboron compounds, palladium(II halides) transmetalation is a critical step.
Boronic acids have been prevalent compounds in Medicinal Chemistry due to their general characteristics, various biological applications, and potential molecular targets.
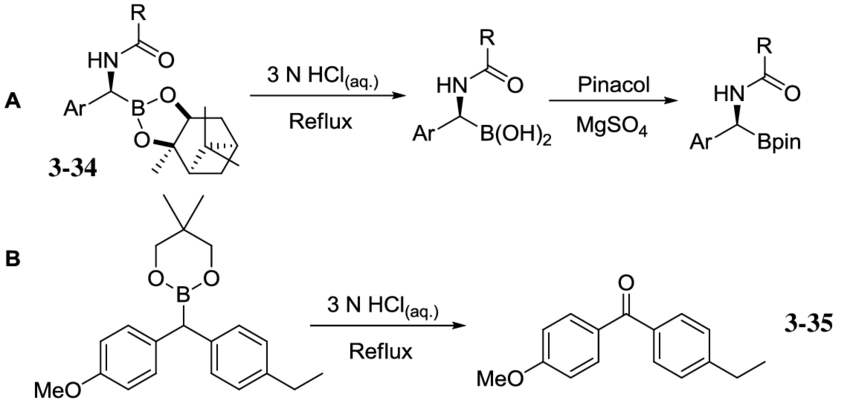
Boronic Esters
Pinacol, neopentyl, and catechol boronic ester are the most widely used boronic esters for SM coupling. It is because of their relative cost, stability, reactivity, and ease of preparation compared to the wide variety of other boronic ester options.
The s-donating power of carbon means that the lone oxygen pairs in boronic ester are more easily conjugated into the electron-deficient boron center. Boronic esters are generally less reactive than boronic acid due to this reduction in Lewis acidity. In most cases, they are stable towards column chromatography, which helps in their isolation and purification. Many are also liquids that can be easily distilled at room temperature. Boronic esters dissolve quickly in apolar solvents. They are neither hydrogen bond donors nor able to be oligomerized like boronic acids. This makes them monomeric.
Boronic esters exhibit more excellent chemical stability than their corresponding boronic acids, but it is not clear what the active transmetalation species is during their SM coupling – suzuki coupling.
Boronic Esters
Boronic Esters
Source: https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.researchgate.net%2Ffigure%2FScheme-3-43-Transesterification-of-boronic-esters-A-Suginomes-hydrolysis-and-pinacol_fig13_267415709&psig=AOvVaw1nyrquVMXOsmOBCJNCnW9c&ust=1639205918561000&source=images&cd=vfe&ved=0CAsQjRxqFwoTCJDr-ofU2PQCFQAAAAAdAAAAABAD
Application of Suzuki Coupling Boron Compounds
Today, it is rare to find a total synthesizer that doesn’t involve at least one cross-coupling reaction. In particular, a Suzuki Miyaura reagent.
Similarly, the B-alkyl SMC was utilized in the synthesis of beneficial products. The fungal metabolites Cytochalasin Z8 and Ieodomycin D have diverse biological activities targeting cytoskeletal processes. Scheme 16 also includes examples of complex molecules that were achieved by synthetic routes involving SMCs with C(sp2)–B reagents, namely Michellamine (an anti-HIV viral replication receptor) and (-)-steganone (an antileukemic lignan precursor).
The potential for borohydride reagents in synthesis has been recognized since the 1940s. This area of chemistry was only really able to take off after the Suzuki-Miyaura coupling reactions were discovered in the late 1970s.
Many boron compounds have found applications in medicinal chemistry and materials science. For example, α-aminoboronic acids can form dative bonds with nucleophilic amino acid residues such as serine and threonine, which are found in the active site of proteases. These reversible dative bonds are more potent than non-bonding intermolecular interactions. The best-known example is the anticancer drug bortezomib (marketed as Velcade), approved by the Food and Drug Administration (FDA) in 2003 to treat relapsed or multiple refractory myelomas. Multiple myeloma is a cancer of plasma cells. Bortezomib has the distinction of being the first approved proteasome inhibitor as well as the first approved boronic acid-based drug on the market.
The discovery that boronic acids can inhibit proteases has led to the approval of boronic acid-centered pharmaceuticals to treat several diseases. Three boronic acid-based drugs (bortezomib, ixazomib, and tavaborole) have been approved for human use since 2003, with many more in the pipeline.
Furthermore, the boronic acid-based materials are currently being studied as potential probes for critical biological molecules such as saccharides, glycoproteins, and catecholamines, as well as complex anions such as fluoride. These developments will undoubtedly spur further research in organoboron chemistry in the upcoming years. Many new exciting discoveries in their applications (including in synthesis) will be made as a result.





