Boron Trichloride
Boron trichloride is an inorganic compound with the formula BCl3. It is a colorless gas that is highly reactive to water. Common uses for Boron Trichloride are in organic synthesis. Boron Trichloride does have several negative health effects, so it needs to be handled with care.

Boron Trichloride
Chemistry of Boron Trichloride
Boron trichloride (Bcl3) is an inorganic chemical compound with the molecular formula BCl3 and a molecular weight of 117.170g/mol. It is a colorless gas with a pungent odor and is a widely used reagent in organic chemistry.
Boron Trichloride Structure
The boron molecule structure has a triangular shape, which is the result of a 120-degree bond angle. While it doesn’t form a full octet, it is non-polar. To have a positive charge, a non-polar molecule must undergo an asymmetric shapeshift of its electron density. Hence, the boron trichloride molecule has no dipole moment. Moreover, it forms three ssp-p bonds with three chlorine atoms.
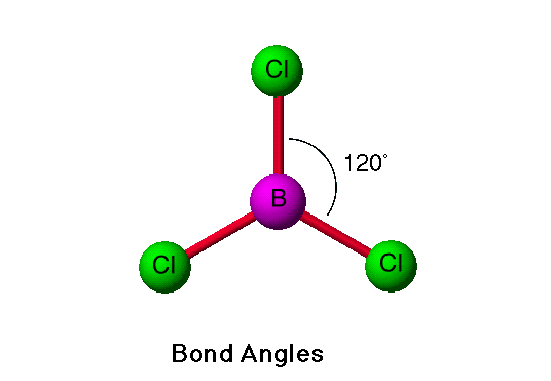
Boron Trichloride Structure
Source: Chemedx
The chemical composition of boron is a complex one, with the most common form of the element being a trigonal mineral. Borates, on the other hand, are Lewis acids.
When exposed to a Lewis base, the trigonal boron atom accepts a pair of electrons and adopts a tetrahedral configuration, which meets the octet rule. In complex borates, boron trichloride and trigonal boron atoms can coexist.
The molecule’s molecular geometry is trigonally planar, with 120-degrees-apart electrons. It is nonpolar due to the central boron atom, which is electron-deficient. This makes the molecule a Lewis acid. Another example of a nonpolar molecule is carbon disulfide. It is an ionic salt with a pH of 7.
Properties of Boron Trichloride
Boron Trichloride Properties
Chemical Formula BCl3
Molecular weight 117.17
Boiling Point at 101.3kPa 12.5°C
Melting Point at 101.3kPa -107°C
Critical temperature 178.9°C
Critical Pressure 3.87MPa
Flammability Not applicable
Toxicity Toxic
Appearance Colourless gas
Density 0.004789 g/cm3
| Boron Trichloride | Properties |
|---|---|
| Chemical Formula | BCl3 |
| Molecular weight | 117.17 |
| Boiling Point at 101.3kPa | 12.5°C |
| Melting Point at 101.3kPa | -107°C |
| Critical temperature | 178.9°C |
| Critical Pressure | 3.87MPa |
| Flammability | Not applicable |
| Toxicity | Toxic |
| Appearance | Colourless gas |
| Density | 0.004789 g/cm3 |
Preparation and Synthesis
Boron can react with certain elements to form new compounds. For example, Boron reacts with halogens (such as chlorine) to produce trihalides (boron trichloride). Industrially, it is produced by chlorinating boron oxide and carbon at temperatures around 501°C.
B2O3 + 3 C + 3 Cl2 → 2 BCl3 + 3 CO
The Kroll process for converting titanium dioxide to titanium tetrachloride is similar to carbothermic reduction. By exchanging halogens in the lab, boron trifluoride reacts with aluminum chloride to form boron trichloride. Despite NMR examinations of mixtures of boron trihalides indicating the presence of mixed halides, it does not dimerize.
Reactivity
- Boron trichloride is an energetic compound that sparks when mixed with fat, grease, and nitrogen peroxide or phosphine.
- In the presence of water, it can also react violently to release heat, followed by hydrogen chloride fumes (hydrochloric acid) and boric acid.
- Boron trichloride and aniline interact violently without cooling or a diluent.
- The ethyl esters of hydroxy acids react with boron trichloride to form the ethoxy carbonyl-substituted boranes in 80-85% yield.
- Borates, chloroborates, and dichloroboronites are produced when boron trichloride is reacted with chloroalcohols which are disproportionate when heated.
- With pyridine and nitrobenzene, it forms additional compounds.
- In the presence of water, it degrades a wide range of metals.
- Ammonia reacts aggressively with boron halides.
- Active metals, metal hydrides, and hydrogen decrease boron halides.
- Elemental boron and the metal halide are produced by reacting alkali and alkaline earth metals at high temperatures.
- Water, lower alcohols, hydrogen sulfide, alkyl mercaptans, ammonia, primary and secondary amines, phosphine, and arsine all react with the trihalides and release hydrogen halide in each case.
Source: https://webwiser.nlm.nih.gov/substance?substanceId=525&identifier=Boron%20Trichloride&identifierType=name&menuItemId=7&catId=64#:~:text=Boron%20trichloride%20reacts%20energetically%20with,hydrochloric%20acid)%20and%20boric%20acid.
Reduction
Boron trichloride can be converted to diboron tetrachloride by heating with copper metal in the laboratory.
2 BCl3 + 2 Cu → B2Cl4 + 2 CuCl
In the solid-state, colorless diboron tetrachloride (m.p. -93 °C) is a planar molecule (similar to dinitrogen tetroxide), but the structure is staggered in the gas phase. At room temperature, it decomposes into a sequence of monochlorides with the general formula (BCl)n, where n can be 8, 9, 10, or 11. The compounds with formulas B8Cl8 and B9Cl9 show closed cages of boron atoms.
How Boron Trichloride is Produced
Important uses of boron trichloride are in organic synthesis. Its strong water reactivity makes it a valuable reagent for organic synthesis. It is produced industrially at temperatures of about 500 degrees centigrade through direct chlorination of boron oxide.
This process is analogous to the Kroll process. It can also be produced by halogen exchange. It is packaged as a liquid and shipped in bulk to semiconductor manufacturing facilities.
Global Boron Manufacturers
The global boron trichloride industry is highly concentrated, with only limited countries involved in the business. The major manufacturers of this chemical are mainly found in the United States, Europe, Japan, and China; however, its market is growing as more people turn to this chemical for its uses in electronics and pharmaceuticals.
Global Boron Trichloride Market
Boron Trichloride is a necessary chemical for many industries and processes. Its demand is growing by its increasing use in the semiconductor industry for plasma etching and gas for CVD. It is also used as an important raw material for pharmaceutical and agrochemicals, catalysts, boron nitride (BN), and other applications.
In 2022, the Global Boron Trichloride Industry is expected to rise at a CAGR of 4.9 percent during 2021-2026, from USD 252.5 million in 2020 to USD 336.5 million in 2026.
Applications
Boron trichloride is a versatile chemical that can be used in many different industries. It has various applications, such as being the starting material for producing elemental boron and playing an important role during the refining processes of aluminum, zinc, and copper alloys to remove oxides and nitrides from the molten metal.
Another common use case involves improving aluminum casting quality by treating melts with the boron trichloride vapor.
BCl3 can also be used to create a uniform and long-lasting adhesive carbon coating over a ceramic basis in the manufacturing of electrical resistors. It has been utilized as a source of boron to increase the BTU value in high-energy fuels and rocket propellants.
Semiconductors
Boron Trichloride is used in semiconductor production in a process known as plasma etching, a $200 million dollar industry.
As the cost of boron trichloride has increased, it is increasingly difficult to procure. This compound has the potential to meet the high purity requirements required by semiconductors. It can also be used to produce displays for electronic products.
Boron Trichloride as a Soldering Flux
Boron trichloride is a non-hygroscopic organic compound that improves the solder spreading the power of soldering flux. This chemical compound is also non-corrosive. In comparison, zinc chloride is non-hygroscopic, but it does leave a residue. The three major boron trichloride soldering fluxes are zinc chloride, boron trifluoride, and sulfate of lead.
In addition to its excellent metallurgical properties, boron trichloride can also be used as a filler metal. The composition of boron trichloride fluxes varies according to their composition. Depending on the amount of boron trifluoride in flux, it may consist of as much as 50% of a non-uniform organic substance.
Harmful Effects of Boron Trichloride
BCl3 is a reagent that can form dangerous hydrogen chloride when exposed to moisture or alcohol. The dimethyl sulfide adduct (BCl3SMe2), which is solid, is safer to use however, water will destroy the BCl3 part while leaving behind dimethyl sulfide in the solution.
Several types of toxicity have been associated with boron trichloride. Boron trichloride needs to be treated with care and respect. It is extremely toxic. It can be harmful if not handled properly. Exposure can lead to severe burns and eye damage. It is also suspected of damaging fertility and the unborn child. Prolonged exposure can cause organ damage.
In animal studies, boron tribromide was the most toxic, followed by hydrogen chloride and bromide. Compared to boron tribromide, these three compounds were equally toxic to rats. In a 2005 report, the American Conference of Governmental Industrial Hygienists documented biological exposure to borate compounds. These compounds were a significant source of environmental toxicity, particularly in wastewater treatment facilities.
Boron Trichloride is Corrosive
As a colorless gas, Boron trichloride irritates the eyes and mucous membranes. It is highly corrosive to metals and tissue,. When exposed to intense heat, boron trichloride containers may explode, causing injuries.
Boron trichloride is a corrosive chemical with strong reactivity toward several metals, including lead, graphite-impregnated asbestos, sodium, and potassium. It also attacks elastomers and packing materials, such as natural and synthetic rubber. It also attacks metals when exposed to moisture. The gas is highly flammable and must be handled with care.
Because of its corrosive nature, boron trichloride should be handled with extreme caution. The fumes from boron trichloride are toxic to the eyes and mucous membranes.
Contact with boron trichloride causes severe skin burns, and it can even cause scarring. Boron trichloride is also very corrosive to metals and can cause electrolyte depletion in electrolyte-rich body fluids.
If exposed to boron trichloride, patients should remove themselves from the affected area and seek medical attention. In severe cases, patients should seek medical attention immediately.
If the burns or other symptoms are caused by a drug misusage, they should gargle and drink water and be sent to a hospital. Inhalation of toxic smoke can lead to emphysema or even death.
Boron Trichloride Causes Lung Oedema
If you have been exposed to boron trichloride, you are at risk of developing noncardiogenic pulmonary oedema, chemical pneumonitis, and skin burns.
The gas produced from boron trichloride exposure can cause chemical and respiratory burns. The substance can also cause severe skin irritation and even gastrointestinal bleeding.
The most common way to avoid exposure to boron trichloride is to stay away from a location where this substance is stored. It is best to store containers of this substance in a cool, dry place and use a chemical fume hood.
Storage of Boron Trichloride
The Safety Data Sheet (SDS) for boron trichloride contains safety recommendations for safe handling. Recommendations include use of a chemical fume hood approved by the REM and one with a face velocity of 80 to 125 feet. Boron trichloride must be kept away from acids, oxidizing substances, and moisture.
Another method is to use protective clothing and equipment to protect the lungs from this toxic gas. Wearing protective clothing is always recommended, as a contact with this substance may cause skin burns and vision loss.
In addition, contact with boron trifluoride in the eye can cause irritation and eye damage. Contact lenses should be removed immediately and eyes should be flushed with copious amounts of water.





