Potassium Borates
Potassium Borates in salt form is a naturally occurring mineral. The ionising properties of borate salts can inhibit protein synthesis and disrupt the bacterial cell membrane. This can affect growth and function. This mineral can be toxic to humans and pets. It can also be used as a disinfectant. There are many uses for potassium borate salts.

Potassium Borates
What are Potassium Borate Salts?
Potassium borate salts are crystalline compounds with a definite molecular structure. When mixed in scrubbing solutions, the molecule of potassium-magnesium borate is 0.84 per cent of the reagent.
Potassium borate salts are available in different forms. The most common conditions are potassium metaborate (K₂B₂O₄), potassium tetraborate (K₂B₃O₇), and sodium tetraborate (Na₂B₄O₄.4H). The other states are sodium tetraborate, potassium tetraborate tetrahydrate, and potassium tetraborate decahydrate. These are the most common forms.
How are Potassium Borates Prepared?
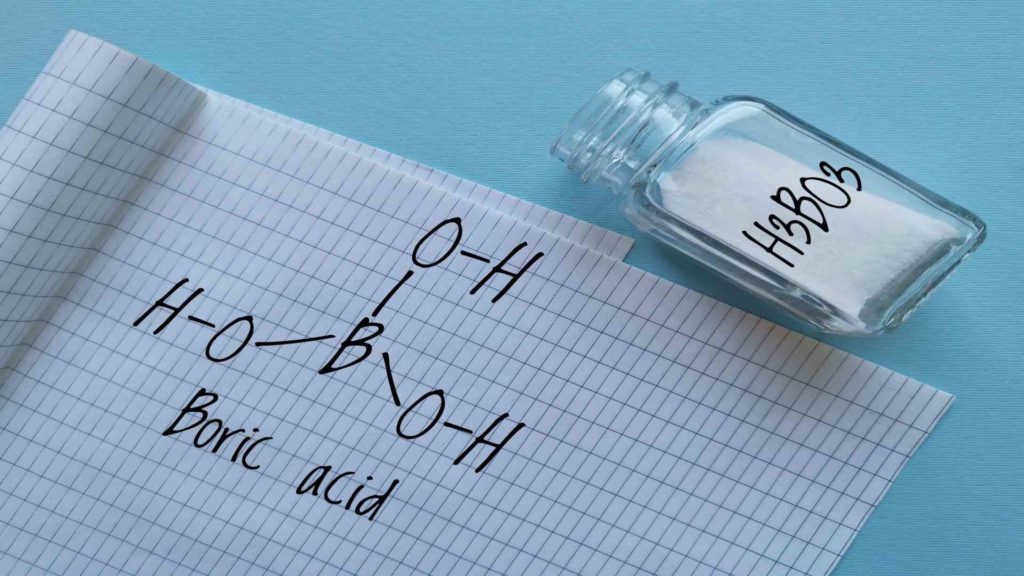
It is well known that potassium tetraborate produces a controlled reaction between potassium hydroxide, water, and boric acid. The salt is an alkaline salt with excellent buffering properties and white crystallised granules. An alkali borate is required, but sodium salts are not suitable; potassium tetraborate replaces borax. Moreover, potassium tetraborate is more soluble in water than boron alone.
A mixture of potassium-magnesium borate and boric acid can be prepared. A combination of these two can be as much as 10% of the total weight of the solution. A few milligrams of the material can make the mixture completely saturated. The result is a highly effective product with a higher concentration than the natural solution. This substance is an ideal agent for reducing pain and other symptoms associated with arthritis.
Applications of Potassium Borates
Potassium borate salts are commonly used as an antiseptic and disinfectant in scrubbing solutions. Their pH is neutralising and increases with concentration and temperature. This mineral is widely used in detergents and special metal fluxes and can be made from the aforementioned compounds. They are soluble in water and can be extracted by distilling the resulting solution. However, potassium borohydride is more expensive than alternatives. Using pure minerals is preferred.
A variety of potassium borate salts are used in medicine. Sodium borate is used for pain relief. While potassium borate is a mineral used in cosmetics, it is not toxic to humans. It is available in a wide range of concentrations in most pharmacies. They are helpful for a variety of applications. Soluble potassium borate is also an excellent diuretic.
Another common industrial application of potassium borate salts is in glass and vitreous materials. It is an essential component of these materials. It is synthesised from the mineral KBO₂. This chemical is obtained from the reaction of borate with KOH. Besides, it is also a very effective antioxidant. In addition to being a powerful solvent, potassium borate is a valuable additive for water-based paints.
Potassium borate is a common ingredient in ceramics. It can be applied to plastics, rubber, and paper. These chemicals can also be used in paints and varnishes. A chemical boronate is a handy tool in many industrial settings. It is available for sale at most chemical stores.
Potassium Borates are also used to crosslink polymers and specify them. The most common application is as a crosslinking agent. They can be crosslinked polymers and proppants. The best-refined borates are soluble in water, not deterring the polymer from crosslinking.
The composition of potassium borate salts is highly variable. In one study, the compound was made by mixing potassium magnesium and boric acid. The two substances were combined to form a new salt in the following survey. The ion-exchange reaction produced the ion-insoluble boronate. These two compounds are similar, and they are not toxic. Moreover, they do not have any side effects.