What Is Trimethyl Borate?
A member of the class of Borate Ester, Trimethyl Borate is a colorless liquid that ignites with an impressive green flame. Its power to aid the preparation of sodium borohydride and act as a weak Lewis acid (AN = 23) is well respected among chemists worldwide. Borate ester, prepared by heating boron oxides and alcohols under dehydrating conditions, is a popular reagent in organic chemistry.
Borate
Trimethyl Borate Synthesis
Trimethyl borate is a boron triester with a single boron atom and three methoxide groups. It can be made by mixing a large amount of dry methanol with boric acid, boron oxide, and a tiny amount of sulfuric acid, and heating the mixture to dehydrate it if necessary.
Due to the extra methanol used, the finished product would be an azeotropic mixture of methanol (25%) and trimethyl borate (75%). Pure trimethyl borate can be obtained by converting methanol to trimethyl borate with a boron trihalide, such as boron tribromide. However, the trihalide should be added gradually to avoid hydrolysis of the previously present boron tribromide.
It is an essential reagent in organic synthesis because it acts as a precursor to boronic acids. These boronic acids, used in Suzuki couplings, are made by reacting trimethyl borate with Grignard reagents.
B(OCH3)3 + ArMgBr → MgBrOCH3 + ArB(OCH3)2
ArB(OCH3)2 + 2 H2O → ArB(OH)2 + 2 HOCH3
Physical Properties
Trimethyl Borate Specifications
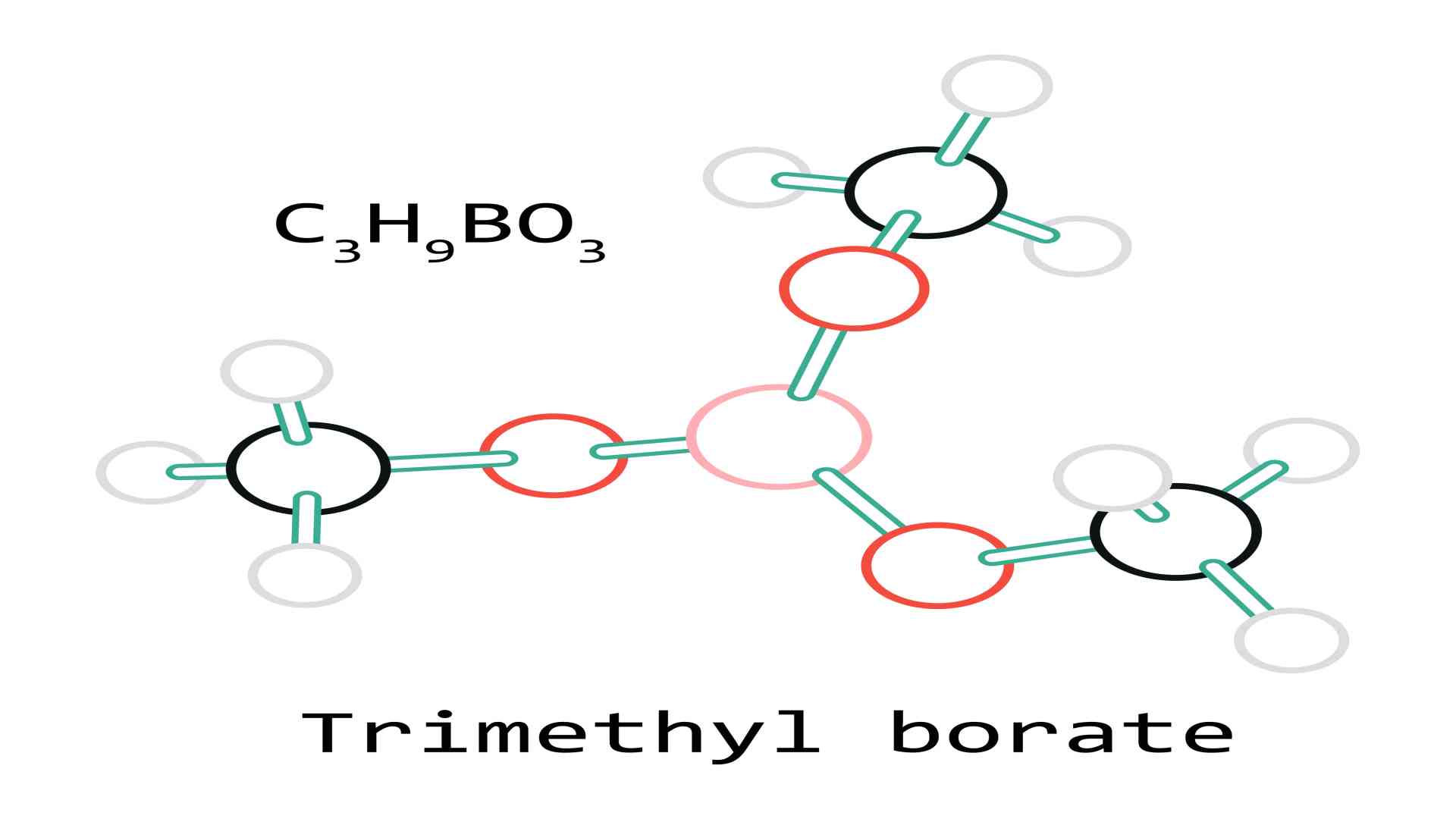
Chemical formula C3H9BO3
Molecular Weight 103.91 g/mol
Density 0.932 g/cm3
Melting Point -34°C
Boiling Point 68°C
Appearance Colorless liquid
Vapor Pressure 137 mmHg at 25 °C
Solubility in Water Yes
Source – Wikipedia
Chemical Properties
Dehydration produces trimethyl borate, which decomposes to methanol and boric acid when coming in contact with water. In the presence of oxygen, it burns to generate boron trioxide. It emits a vivid green hue in flames, which overpowers other flame colours.
Uses for Trimethyl Borate
(B(OCH3)3) is also an anti-oxidant in the brazing and solder flux and has been explored as a fire retardant. Additionally, it has been examined as an additive to some polymers.
It is the main reactant in the Brown-Schlesinger method of producing sodium borohydride, and it is successfully recreated from sodium metaborate (NaBO2) via a sequential process that includes reacting with sulfuric acid, cooling crystallization, and reactive esterification distillation.
The metaborate is first transformed to boric acid (H3BO3) by treating it with sulfuric acid, obviating the need to synthesize borax (Na2B4O710H2O) in traditional techniques. Boric acid is separated and purified from coexisting sodium sulphate by cooling crystallization (Na2SO4).
Following that, it is made by esterifying boric acid with methanol, with reactive esterification distillation used to speed up the process and purify the product. X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), and gas chromatography are used to demonstrate the formation of boric acid and trimethyl borate (GC). Sodium metaborate could be converted to boric acid at a rate of about 55%, with a manufacturing yield of 74.1 to 96.5% for trimethyl borate esterified from boric acid as-produced.
Trimethyl Borate for Protection of Wood-based Materials
B(OCH3)3 has a high vapour pressure injected into a container containing the wood beneath a vacuum during vapour phase treatments. It then volatilizes and diffuses into the wood by combining with any moisture to form methanol and boric acid. The borate is released during the reaction and is deposited in the wood. Some methanol and borate remain in the wood when the vacuum is released. This method has been used to cure a variety of composites, but its utility is limited because the wood being processed cannot be too wet (moisture content less than 6–8%).
Risks of Trimethyl Borate
- When inhaled or absorbed via the skin, it can negatively influence.
- It irritates the nose, throat, and lungs and causes headaches, nausea, lack of appetite, vomiting, diarrhoea, and convulsions.
- The liquid is highly combustible, posing a fire risk.
- If ingested, it might harm the kidneys.
Precautions for Safe Handling
- Goggles should be worn when burning trimethyl borate to prevent boric oxide particles from stinging the eyes.
- It should only be kept in well-sealed bottles away from light and heat. It’s important to keep the bottle sealed or place it in a desiccator because it’s sensitive to water.
- Individuals who have been advised of the dangers of trimethyl borate exposure should wash contaminated work clothing.
- Eyewash foundations should be available in the workplace in case of an emergency.
- When encountered on the skin, wash or shower right away to get rid of the chemical. Wash every body part that has come into touch.
- Do not eat, smoke, or drink where this type of borate is handled, processed, or stored.





