Understanding the Bohr Model
Let’s clarify things and show you how important this model is for scientists. So, according to Niels Bohr, let’s start understanding the mysterious world of atoms.
The Bohr Model, developed by Niels Bohr in 1913, revolutionized our understanding of atomic structure.
The Bohr model is just like the planets orbiting around the sun in the solar system. It is one of the most important scientific discoveries, as it laid the groundwork for further breakthroughs in understanding the atomic structure and its implications for physics and chemistry.
This work’s success lies in its ability to justify Rydberg’s formula for hydrogen spectral series, which was never experimentally confirmed before. It proposed that the electrons revolve around the nucleus of an atom in well-defined circular paths, known as orbits or shells.
Each orbit or shell has a fixed energy and is known as the orbital shell or energy level. These orbital shells are identified by an integer, n, also known as quantum numbers. The first orbital shell, n=1, is assigned as K, n=2 as L, n=3 as M, and so on.
Electrons in the lowest energy level, n=1, are considered to be in the ground state. To move from lower to higher energy levels, electrons gain energy, while they lose energy on moving from higher to lower energy levels.
Let us understand this in detail with the example of the Bohr Atomic Model of Boron.
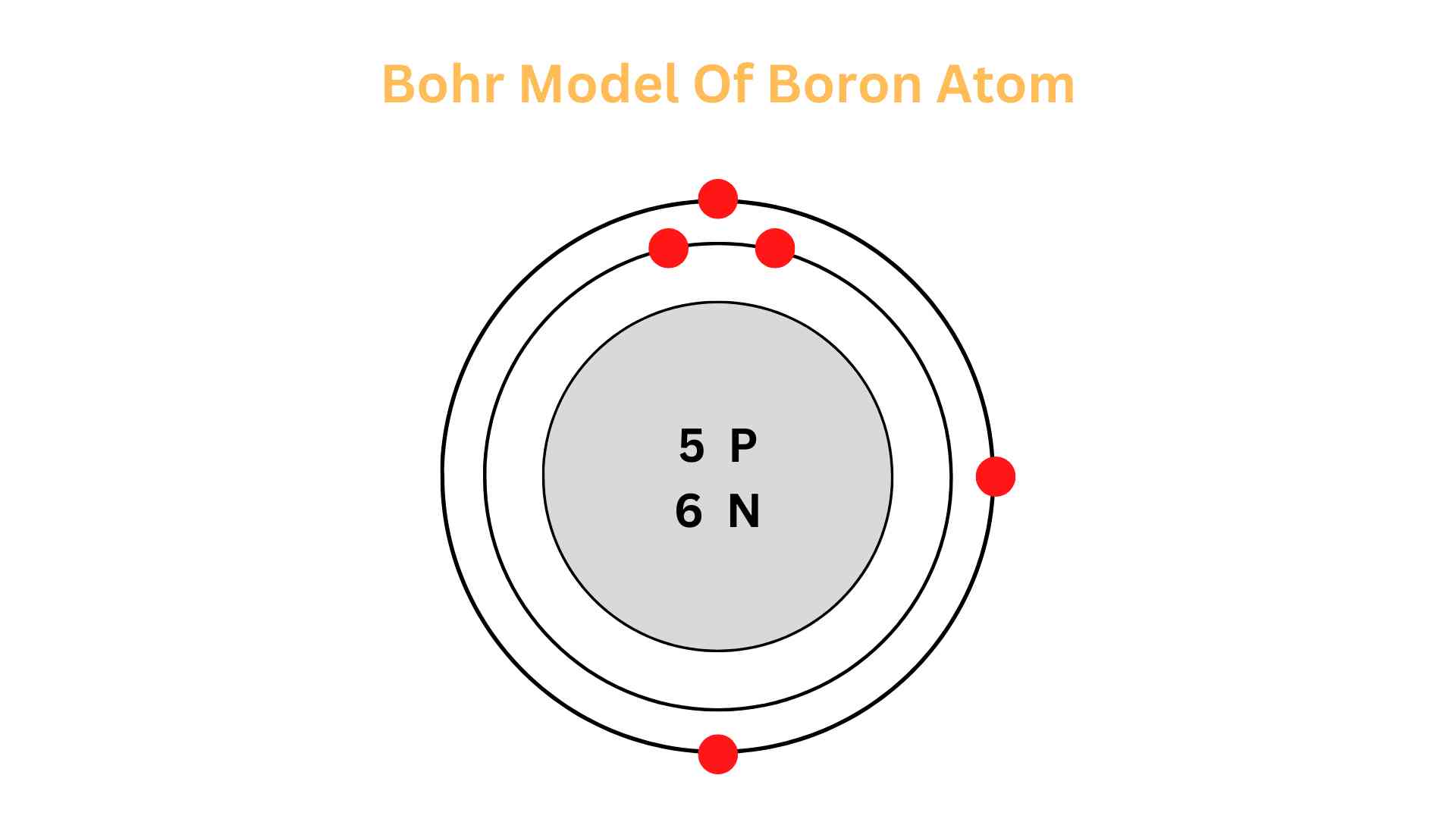
Boron is a fascinating element. Its nucleus contains 6 neutrons and 5 protons, ringed by two electron shells named K-shell and L-shell. The outermost shell holds 3 valence electrons which are essential for understanding the chemistry of Boron.
To understand how to draw the bohr model of boron, let’s go through the steps with this helpful diagram.
Step 1:
Count the protons, electrons, and neutrons. Boron has an atomic number of 5 and an atomic mass of 10.811, which means it has 5 protons, 5 electrons, and 11 neutrons.
Step 2:
Sketch the nucleus, which is a small circle made up of the number of protons and neutrons.
Step 3:
Draw a circle around the nucleus, i.e., the first orbital shell; K. K holds a maximum of two electrons. The boron atom has 5 electrons, so place two electrons from it in the K shell.
Step 4:
Draw the second orbital shell; L. L holds a maximum of eight electrons. So, place the remaining 3 electrons in the L shell. In this shell, electrons are placed one at a time, beginning from the top position and going clockwise.
Orbital shell Bohr model
This completes the Bohr model of the boron atom. It has 5 protons and 6 neutrons in the nucleus and 5 electrons orbiting it, two in the K shell and three in the L shell.
Bohr model atom
The Bohr model of the atom has proven to be a breakthrough for scientists, as it explains the structure of atoms and how they work.
It also enables us to understand more about the composition of matter and energy.





