Boron Electron Configuration
Boron is a chemical element with the chemical symbol B. Boron’s atomic number is 5. Boron has an electron valence of 3, meaning it has 3 electrons within its valence shell. Its atomic configuration is1s22s22p1. It is concentrated on Earth by its property of water-solubility of the naturally occurring compounds, such as borate minerals. They are mined by the industrial process as evaporites, like kernite and borax. Elemental boron, a metalloid that is chemically uncombined, is not naturally found on Earth. There are many allotropes of boron. Amorphous boron, a brownish-colored powder, and crystalline boron, which is black-colored and silvery, exist. Boron is used primarily for the manufacture of various industrial, household, and agricultural products.
Writing Boron’s Electron Configuration
To understand boron’s electronic configuration, one must first know the number of protons, neutrons, and electrons and understand the related basic concepts. Further, this allows us to know why and how boron has more than one definite electronic configuration.
Protons and neutrons in Boron
Boron has the atomic number 5. This means that it has 5 protons in its nucleus. The atomic number of an atom is the sum of all protons found in its nucleus. It is also known by the symbol Z.
The neutron number of an atom refers to the total number of neutrons within the nucleus. It is also known as the neutron number of an atom. This symbol is N. The neutron number plus the atomic number equals the atomic mass number: N+Z=A. There are usually many stable isotopes for stable elements. Isotopes can be nuclides with the same atomic numbers but differ in the number of neutrons. Mass numbers of typical isotopes of boron are 10; 11.
Electrons in Boron
An electrically neutral atom will have the same number of electrons as the number of protons in the nucleus. The number of electrons found in the boron’s neutral atom is therefore 5. Every electron is affected by the electric fields created by the negative electrons (Z – 1) and the positive nuclear charges.
The chemical behaviour of atoms is determined by the number and arrangement of electrons. Therefore, the atomic number is used to identify the different chemical elements. These electrons are organized according to quantum mechanics principles. The main factor in determining the element’s chemical bonding behavior is the number of electrons within its electron shells.
Boron’s Main Isotopes
There are 13 isotopes of boron. The shortest-lived one is 7B, which decays via proton emission or alpha decay. It has a half-life of 3.5×10-22 seconds. Boron has two stable and naturally occurring isotopes: 11B (80.1%) and 10B (19.9%).
Boron-10 is made up of 5 protons and five neutrons, as well as 5 electrons. Boron can be used to absorb neutrons due to its high neutron cross-section in isotope 10B.
Boron-11 is made up of 6 neutrons and 5 protons. Absorption cross-section of 11B for thermal neutrons is approximately 0.005 barns (for 0.02 eV neutrons).
Boron Electron Configuration
The electron configuration of boron can be written according to the Aufbau principle as follows: the first two electrons go in the 1s orbital. Now the 1s orbital can only hold 2 electrons, so the two next electrons of boron go in the 2s orbital. The 2p orbital will hold the remaining electron. The B electron configuration will therefore be 1s22s22p1.
There are many possible oxidation states: -5; 1; +1; +2 and +3. Boron does not normally react with acids. It reacts with both hot nitric and hot sulfuric acids (HNO3) in powder form. It can also be dissolved in molten (melted) metals. Boron is the most well-known compound. It has the formal oxygenation state III. These are oxides, sulfides, and halides. As a catalyst, boron trifluoride can be found in the petrochemical sector. Boric acid is formed when the halides react with water. Boron can be found almost exclusively in nature as different oxides of B(III), which are often associated with other elements. Borane minerals that contain boron in the oxidation state +3 are more than 100.
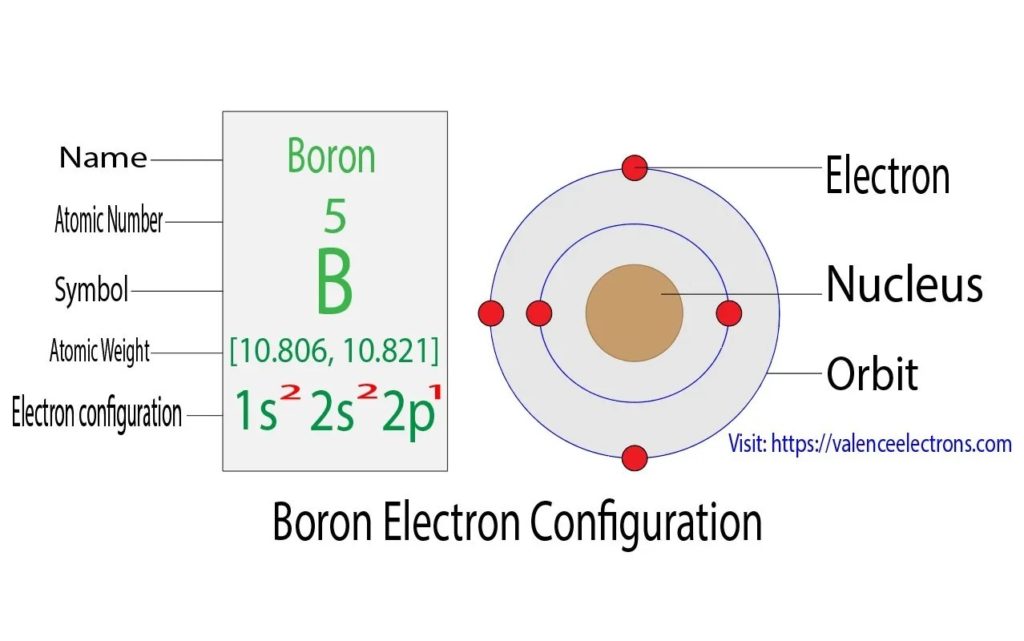
Diagram – Boron electron configuration – Source: https://valenceelectrons.com/boron-electron-configuration/
Other Electron Configurations of Boron
Boron contains 3 electrons in the valence shell of its element, i.e. its atomic configuration is 1s22s22p1. The 2s and the 2p valence electrons have been designed to be hybridized to give three hybrid orbitals with sp2 orbitals. In a first approximation, boron forms three covalent bonds to get BH3, BX3, and BR3. This is only a rough approximation. Boron has been shown to exhibit electron-deficient bonding. This is evident in the “3-center, 2 electron bonds” between hydrogen and boron which is discussed in detail ahead.
Many compounds of boron have an oxidation state that is +3. However, the first three ionization energies for boron are too high to allow for the formation of compounds containing B3+. Therefore, in all its compounds boron is covalently bonded. This means that one of the 2s electrons in boron is made into a 2p orbital. The outer electron configuration 2s12p2 is created.
The s and the p orbitals can then be combined to create sp2 or sp3, allowing boron to be four- and three-coordinated, respectively. Three-coordinated derivatives (e.g., halides, alkyls, and aryls) are planar molecules that can readily form donor-acceptor compounds (called adducts), with compounds containing lone pairs of electrons.
The boron atom in these adducts is four-coordinated, with the four groups being tetrahedrally placed around it. Tetrahedral bonds are formed when a donor atom receives an unshared pair of electrons, either a neutral molecule (or an anion). This allows for a wide range of structures to be formed.
Compounds of boron exhibit the behavior of “electron-deficient” species. This implies that there are not enough electrons to permit the formation of conventional 2-electron bonds. This is why boron can accept protons (H+) in solution. Boron-hydrogen compounds can also be called boranes or boron hydrides.
The simplest hydride, borane BH3, which can be expected to have a planar triangular structure, utilizes trigonal sp2-hybrid atomic orbitals (which can be designated tr1, tr2, tr3). It is an extremely unstable compound, however, and spontaneously dimerizes to form diborane, B2H6. (The fluorine analog BF3 is a stable molecule secured by the larger electronegativity of fluorine.)
In diborane, there are 12 valence electrons. Three from each of the six B atoms and 1 from each of the six H. When this structure is made according to the rules required to draw any lewis structure model, it must have 14 valence shell electrons; however, it does not. The two B atoms and four H atoms lie in the same plane (sp3– perpendicular to the plane of the page). In these four bonds, 8 electrons are involved.
Four electrons bond the remaining H atoms to the two B atoms and the B atoms together. This is done when the two H atoms simultaneously bond to the two B atoms. This creates an atom “bridge” because there are two electrons shared among three atoms. These bonds are also called three-center two-electron bonds. Heavier boranes (hydrides of boron), such as B4H10, B5H9, etc., make extensive use of the theme of B-H-B and B-B-B bridge bonds.
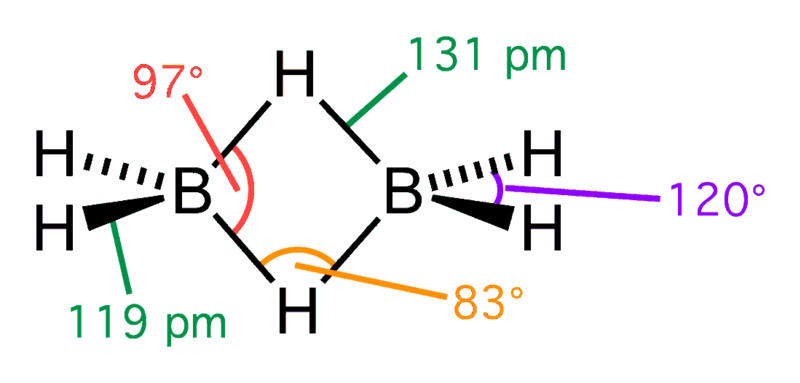
Diborane Structure – Source: Wikipedia





