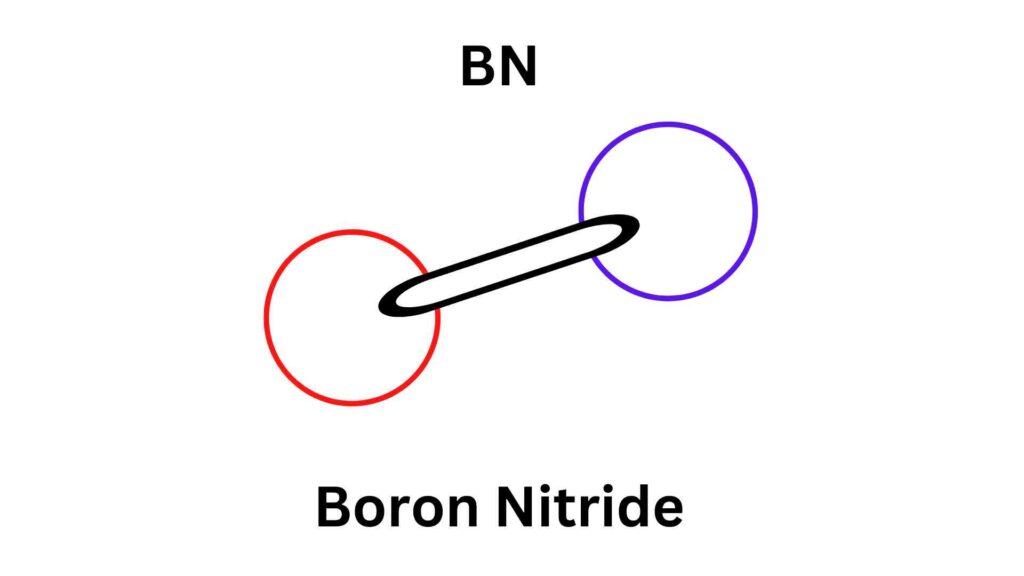
The Bohr Model of Boron: An Overview
The Bohr Model of Boron helps us understand the electron structure and its arrangement around the nucleus of boron atoms. Danish physicist Niels Bohr developed this model in 1913. But what is this Bohr atomic model?
Bohr model
Bohr Atomic Model
The Bohr atom model shows that the electrons revolve in different orbits with varying energy around the nucleus. This model assumes that electrons are similar to planets and the nucleus is like the sun in the solar system.
Also, according to the Bohr model of an atom, an electron’s energy is quantized. It means that electrons can belong to one energy level or another but nothing in between.
The Bohr model of the atom is perhaps the most influential theory in physics because it predicted atomic energy levels and justified them with fundamental physical constants.
The success of this work was mainly due to its ability to explain Rydberg’s formula for hydrogen spectral series, which had never been experimentally confirmed until then.
The Boron Bohr Model
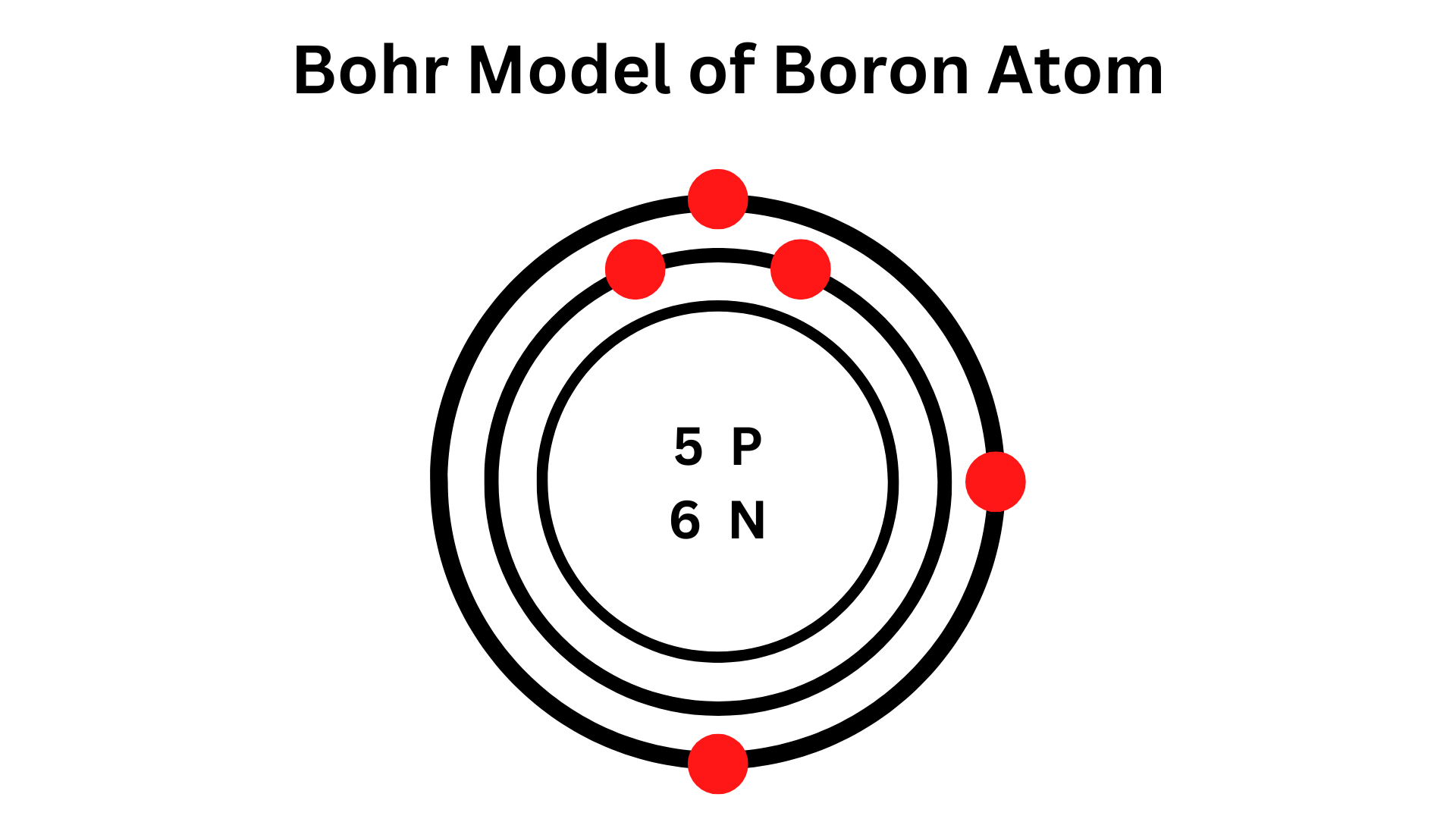
Bohr Model of Boron describes the electrons’ orbit and their visual representation around the tiny nucleus.
It uses various electron shells like K, L, M, N, and others. Each shell holds a particular number of electrons, with the shell closest to the nucleus having the least energy. As the shell goes farther away, the energy levels grow.
The boron Model comprises a nucleus with six neutrons and 5 protons. Two electron shells, namely K-shell and L-shell, surround the nucleus. Additionally, there are 3 electrons in the outermost shell of the Bohr diagram, which are the valence electrons.
Bohr Model of Boron
Boron is a versatile chemical element that can be found in many different compounds. It has an atomic number of five and the symbol B on the periodic table.
Boron also has six neutrons, five protons, and five electrons. The electron shells for this element are divided into two, with two electrons in its K-Shell (I) and three in its L-Shell (II). In total, boron has three valence electrons.
Drawing the Boron Atom Using the Bohr Model
1. Calculate the number of protons, electrons, and neutrons in the Boron atom
- Protons, electrons, and neutrons are all important components of the atom.
Protons, have a positive charge and serve as the atom’s nucleus and the neutrons. The electrons then orbit around this nucleus, giving off a negative charge. Neutrons fill up the nucleus but don’t carry any electrical charge, making them uncharged particles.
- Look at the atomic number of an atom to find the number of protons.
The number of protons in an atom is equal to an atomic number of an atom, i.e., since the atomic number of Boron is five, we have 5 protons in the atom of Boron.
- Find the number of neutrons in an atom using the following formula
The number of neutrons in an atom = Atomic mass of the atom – Number of protons in an atom (N = A – Z)
- Round up the atomic mass to the closest whole number
The atomic mass of Boron is 10.811, rounded off to 11. And the number of protons in Boron is 5. therefore, the number of neutrons in a boron atom is 11 – 5 = 6
- The number of electrons in any neutral atom equals the number of protons.
Since the Boron atom is neutral, its electrons will equal the number of its protons.
Hence, the number of boron electrons = 5 | Number of boron protons = 5 | Number of boron neutrons = 6
2. The Nucleus of the Boron Atom in Bohr’s Model
The nucleus is a thick and small area consisting of protons and neutrons in an atom. Hence, in Bohr’s model, we have a small circle representing the protons and neutrons of the Boron atom.
An electron shell may be considered an orbit followed by electrons around an atom’s nucleus.”
3. The First Electron Shell
The K-shell is the first electron shell closest to the atom’s nucleus, holding up to two electrons. Since boron consists of 5 electrons, place two electrons in the K-shell.
The boron atom has 5 electrons, so the remaining electrons – 5 – 2 = 3 electrons.
Hence, these 3 electrons should now be used in the next shell – the L-shell of the boron atom.
4. The Second Electron Shell
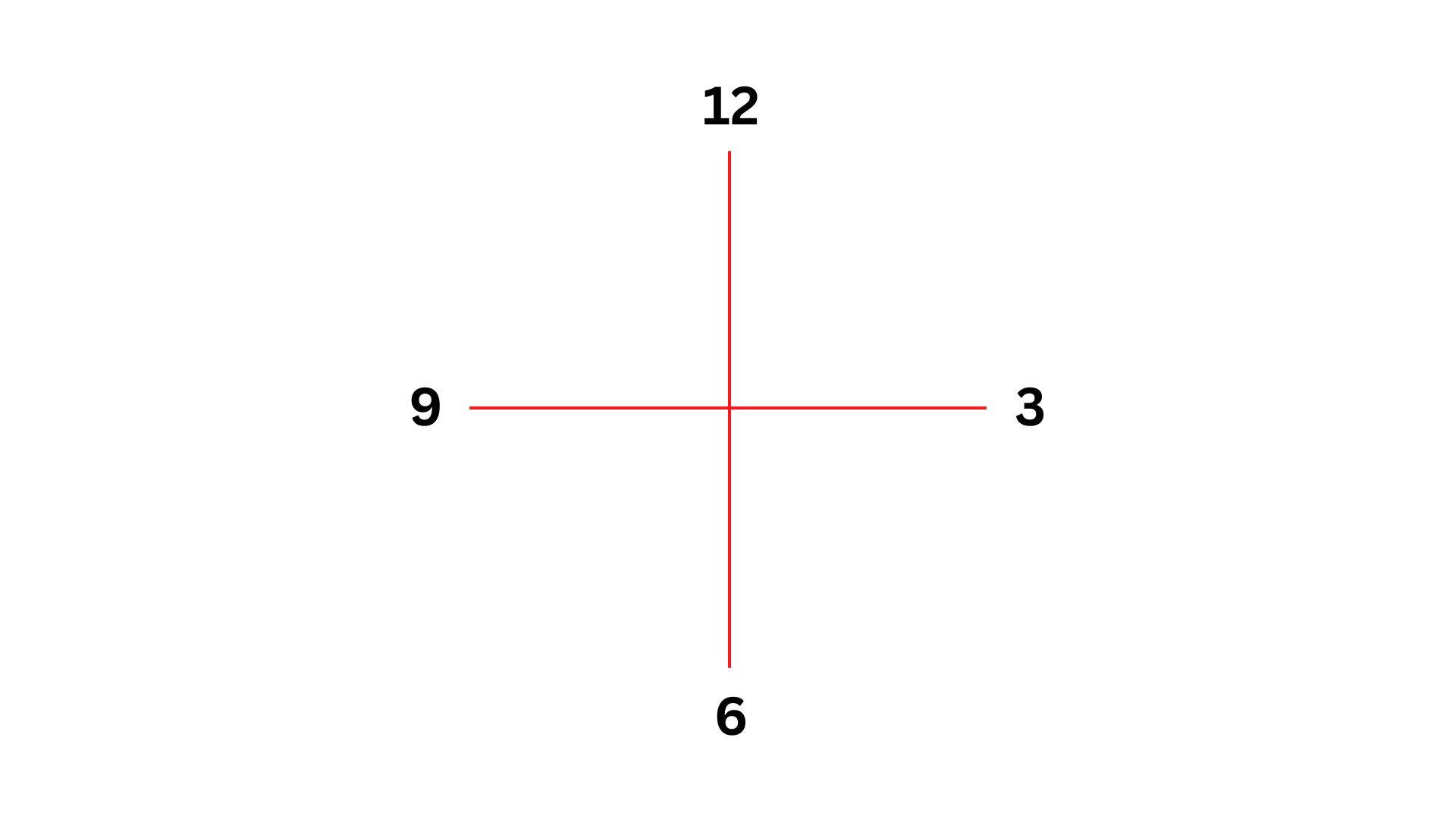
Second Electron Shell
The second shell of an atom in Bohr’s model is called an L-shell. It can hold up to a maximum of 8 electrons. This shell follows the first electron shell.
Since there are already 2 electrons used in the first shell and sticking to the clock position – 12, 3, 6, and 9 o’clock is essential, place the remaining 3 electrons in this L-shell. Begin at the top and go clockwise, placing electrons one at a time.
Unlike the first shell, the second shell can hold electrons in any direction. This is because the first shell is the noble gas configuration. Hence, the electrons in the first shell must be in the nearest noble gas configuration possible.
In the case of the second shell, there are no restrictions so the electrons can be filled in any direction.
However, in the case of the fourth shell, there is a restriction. It is said to be the second noble gas configuration. Hence, the electrons must be in the nearest noble gas configuration possible.
As you move along the periodic table, elements become progressively less reactive. This is because the outermost electrons are filled with more energy and are more tightly held to the nucleus. In this way, the higher-energy electrons are more difficult to remove, leaving the remaining electrons with less energy.
Similarly, the outermost electrons are also the most difficult to remove. This is because they are the most distant from the nucleus and thus have the largest electrostatic repulsion from the positively charged nucleus.
By convention, the electrons are numbered from the top down, with the first shell K-shell associated with the highest energy level and the L-shell associated with the lowest level for 8 atomic numbers.
This completes Bohr’s model of the Boron atom.