Boron-Doped Nanodiamonds For Photocatalysis
What makes boron-doped nanodiamonds so special? Boron-doped nanodiamonds for photocatalyis (BNDs) are a special type of diamond that has been modified with borons. They have been shown to enhance photocatalytic activation and thus increase their potential use in various fields.

Boron Nanodiomand Photocatalysis
Nanodiamonds as Photocatalysts
Diamond nanomaterials are promising candidates for inexpensive photocatalysts. They can be activated by light and then produce carbon-neutral “solar fuels” by speeding up certain interactions between water and CO2. This means that the particles must be exposed to direct sunlight.
The EU project DIACAT is now doping diamond materials with boron and demonstrates how this could enhance photocatalytic characteristics significantly at BESSY II.
Climate change is happening, and it will continue to occur as long as we do not achieve sufficient CO2 emission reductions. One way that we can do this is by returning greenhouse gas CO2 back into an energy cycle, namely through methanol production. However, the process requires energy and catalysts.
If we can harness the energy from sunlight and build light-active photocatalysts made of affordable and commonly available materials rather than rare metals like platinum, there is a chance that “green” solar fuels could be created in a climate-neutral manner.
Can Boron Doping help?
Diamond nanomaterials are not precious crystalline diamonds but tiny nanocrystals of a few thousand carbon atoms that are soluble in water and resemble black slurry or nanostructured carbon foams with large surface areas. They are a great potential as photocatalysts but need UV light for activation. However, this component of the solar spectrum is not very strong.
HZB-scientist Tristan Petit and his cooperation partners in DIACAT have now been working on the modeling of energy levels within such materials, which shows that intermediate stages can be built into a photovoltaic material by doping with foreign atoms.
For example, boron-doped nanodiamonds seem to play an integral role to enhance photocatalytic activation which is mostly attributable to its increased visible light absorption efficiency. Borons addition also creates semiconducting properties, which will soon open up a new class of nanodiamond electronics applications.
Properties of Nanodiamonds
Nanodiamonds are interesting carbon nanoparticles because of their unique physical and chemical properties. Their hardness is higher than other materials. Their chemical stability is better than other materials. And their thermal conductivity is extremely high. So NDs are used in lots of fields, including medicine, electronics, optics, and catalysis. They can also transport small molecules, proteins, and nucleic acids among many other treatments.
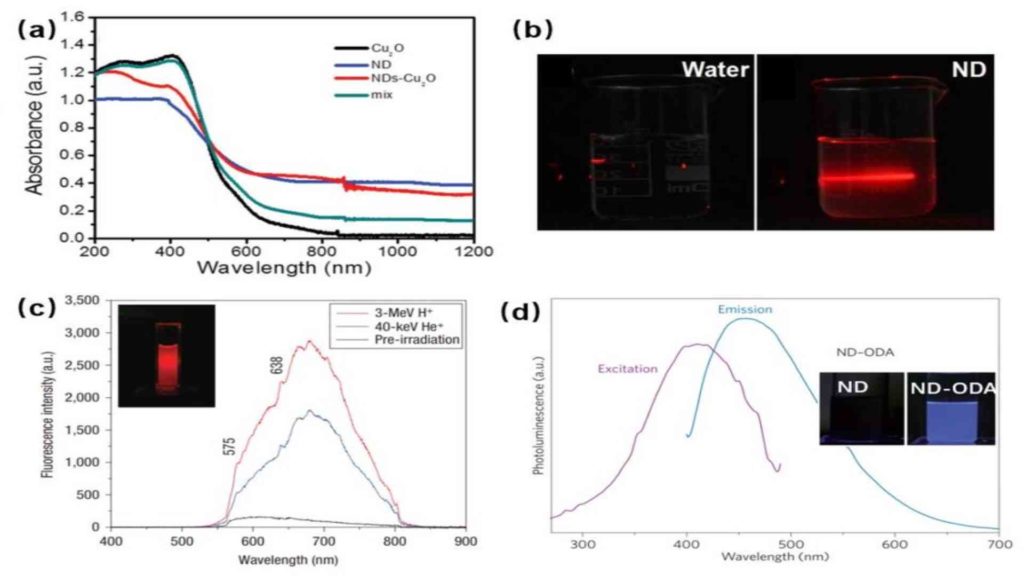
Optical Properties Of Nanodiamonds
Figure 1 shows the optical properties of the NDs.
(A) The absorption spectrum of Cu2O, NDS, NDs – Cu2O, and physical mixtures of NDs and Cu2O
(B) Scattering effect of the NDs in water
(C) Fluorescence spectra in water
(D) Excitation monitored at 450 nm emission (1) Emission at 410 nm excitation (2) Spectra of ND-ODA dispersion in dichloromethane: images of ND and ND-ODA dispersions in dichloromethane with UV (365 nm) excitation.
Application of Nanodiamonds
In the field of photocatalysis, NDs are a newly preferred catalyst compared to TiO2, g-C3N4, or others. NDs were reviewed by focusing on different applications: hydrogen production, air pollution degradation, carbon dioxide reduction, nitrogen reduction, and graphene oxide removal.
These tiny nanoparticles can also be used as antimicrobial agents for their features such as size, shape, and biocompatibility. This makes them ideal for the development of efficient and personalised nanotherapies, such as vaccinations or drug delivery systems.
Nanodiamonds in Bioimaging:
Bioimaging is an exciting area that allows noninvasive insight into the tissues and cells of living organisms to monitor metabolic processes and disease-related alterations. NDs have lately emerged as a good candidate for contrast agents when compared to other nanomaterials. NDs are ideal for bioimaging because of their tiny size, sustained fluorescence, and great biocompatibility.
The NV-center of fluorescent NDs, in particular, is an atom-like light-emitting source, and its electron spins may be detected and manipulated optically even at room temperature. Furthermore, the majority of its emission occurs in the near-infrared range, which is advantageous for biomedical imaging and diagnostics.
Nanodiamonds in Drug Delivery
The biocompatibility, water dispersibility, drug-carrying ability, and targeted therapeutic potential of a nanomaterial determine whether it is appropriate for drug delivery. NDs are modified with specific functional groups so that drugs can physically or chemically be connected to NDs for delivery.
Zhang et al. described a multicomponent NDs-based drug delivery system with simultaneous targeting, imaging, and increased therapeutic capabilities. Anti-HIV medicines based on NDs were delivered to the brain by Roy et al.
Nanodiamonds in Biosensing
A biosensor is a device that analyses biological reactions and converts them into electrical signals. A biosensor is a cutting-edge technology developed by biologists, chemists, physicists, physicians, and electronic engineers. Many advancements have been made in numerous fields since the discovery of NDs. NDs have also achieved some progress in the field of biosensors.
Nanodiamonds in Heat Therapy
Heat therapy is a way of eliminating tumour cells by heating the lesion site to different degrees depending on how sensitive tumour cells and normal cells are to heat. Heat therapy is divided into two categories.
Thermoablation with a treatment temperature control over 47 °C can cause rapid necrosis of tumour tissue under high temperatures, but it also causes harm to normal tissue and requires a complicated clinical trial; hence it is rarely used. The other is hyperthermia when the treatment temperature is kept between 42 and 46 degrees Celsius.
Numerous studies proved that nanomaterials (such as ferromagnetic nanoparticles, metallic nanoparticles, and carbon nanotubes) are capable of hyperthermia therapy for tumour treatment. However, the toxicity of each of these materials varies.
Because of their biocompatibility, NDs have been used in hyperthermia for tumour treatment. Vervald et al. investigated boron-doped NDs for hyperthermic applications in a series of research. In 2016, Vervald et al. explored the influence of boron-doped NDs on hydrogen bonding in plasmonic liquids.
They used a visible wavelength laser to irradiate the boron-doped NDs water dispersion in 2017 and discovered that the boron-doped NDs efficiently heated the surrounding water under laser irradiation.
The ability of boron-doped NDs to heat liquids under the influence of laser radiation opens up exciting possibilities for nano-reagent manufacturing in medical cancer and local thermal therapy.
Potential implications with Nanodiamonds
NDs are environmentally friendly and non-toxic materials that can be used in the design of new biomedical devices. They have remarkable mechanical, optical and fluorescent properties, which makes them highly useful when working with drug delivery or diagnosis approaches.
According to preliminary studies, NDs have the ability to modulate the immune response, which is an important feature to combat viruses. Furthermore, the diverse abilities of NDs make them an excellent candidate for improving medication administration, which, when combined with their fluorescence ability, enables drug monitoring.
Nanodiamonds provide a novel strategy for reducing the high levels of co-morbidity and mortality associated with antibiotic resistance, as well as reducing treatment costs, resulting in reduced antimicrobial resistance.
Some traits that NDs will need in future be robustness against thermochemical changes in their surroundings and permanence in the host’s “hostile environment”.





