Porous Boron Nitride
The formation and applications of porous Boron Nitride have been the subject of popular research lately. Its high porosity and thermal stability will help pave the way for improving large-scale industrial gas and liquid separations, such as those used in carbon capture or water treatment processes by reducing the amount of energy required.

Porous-boron-Nitride–Oil-Spill-Cleanup
Boron Nitride is also helpful for catalysis, hydrogen storage, and oil desulfurization. The Journal of Physical Chemistry cites research by the University of St Andrews and Diamond Light Source. It provides an improved understanding of the formation of porous Boron Nitride and how it can be used for scaling up separation processes.
Adsorption, Adsorbents & their Industrial Applications
Adsorption refers to forming an atomic or molecular layer by a liquid, gas, or liquid. This is significantly different from the process of absorption, where a substance is diffused into a liquid or solid to create a solution. It is a critical industry component and has many uses, including water purification, chemical separation, activated charcoal, synthetic resins, and chemical separation.
As opposed to absorbents and dissolved solids, liquids, or gases, adsorbents are materials that permit a dissolved substance, liquid, gas, or gas to stick to its surface. Adsorbent materials can separate molecules according to their size and chemical composition. They are also less expensive, less energy-intensive, and more sustainable than traditional separation methods.
Because of their large surface areas, porous adsorbent materials are crucial for many industrial applications. The vast majority of chemical separations are responsible for between 10 to 15% of the global energy consumption. This is due to the many processes of distillation, which purify a liquid through heating and cooling.
The porous adsorbent can help minimize energy consumption. Scientists have been creating porous materials with a range of properties and porosities to realize the potential for adsorption separation fully. Porous Boron Nitride is one of them.
What is Porous Boron Nitride
Boron Nitride is structurally similar to activated carbon. Boron nitride can capture large hydrocarbons and other gaseous molecules with a porous structure. It can be used as a lubricant, insulating thermoconductive filler or UV-light emitter. It has several adsorptions and chemical separation applications too.
Recent proof-of-concept studies have shown that it can remove organics from water. The porous structure of Boron Nitride allows it to be used as a drug delivery nanocarrier. Although Boron Nitride’s porosity is challenging to control, it has enormous implications for drug delivery and adsorption performance.
Porous Boron Nitride is an effective adsorbent material because of its high thermal stability. It also has a high surface area. This material has been tested for many applications, including water cleaning, carbon dioxide capture, and oil spill clean-up. Understanding how porous Boron Nitride forms is essential to scale up its synthesis and separation.
Recent Research on Porous Boron Nitride
The Journal of Physical Chemistry C published research by the Petit Group in collaboration with the University of St Andrews, Diamond Light Source, and St Andrews provides an improved understanding of the formation of porous boron nitride, which can be used for scaling-up separation processes.
The team discovered that porous Boron Nitride could only be formed below 700° Celsius. This temperature is crucial to ensure that only boron is formed and not intermediate molecules made at lower temperatures.
Researchers also found that non-porous carbon nitride was formed before porous Boron Nitride formation. This is a crucial step in the formation process, which has not been previously mentioned in other studies.
Anouk L’Hermitte, the lead author of this study, stated that “From our study, we are now able to make greater predictions about both the synthesis process porous Boron Nitride and its adsorption characteristics at large scales.” This will allow industrial processes to be more energy-efficient and sustainable.
This study focused on one specific route to synthesis, with particular reagents (substances used in the chemical reaction), and at a temperature of 1050° Celsius. The following steps include testing the product in different separation situations and trialling the synthesis process with a manufacturer.
Anouk L’Hermitte explained that “our mechanistic study focuses only on a set of synthesis conditions.” The final porous Boron Nitride might differ slightly if one uses different reagents or at a different temperature.
Applications of Porous Boron Nitride
Carbon Capture
CCS (carbon capture and storage) is essential for implementing the Paris Agreement to limit global temperature rise to 1.5 degrees Celsius above preindustrial levels. There are many methods for carbon capture. However, physical adsorption is most efficient due to its high kinetics, cycling ability, and low energy consumption. It is essential to find a high CO2 adsorption potential and selectivity material.
Porous materials make excellent candidates for physical adsorption because of their high selectivity, cost-effectiveness, and high adsorption capacities. Hexagonal Boron Nitride(h-BORON NITRIDE), a porous material, has been a favourite candidate for CO2 adsorption because of its unique properties. These include a large surface area, high pore volume, and polarity.
Water Treatment and Oil Spill Clean-Up
It is vital to remove oils, organic solvents, and dyes from water for water source protection and environmental protection. It is necessary to develop advanced sorbent materials that have high sorption capacities. Study results showed that porous Boron Nitride nanosheets have excellent adsorption properties for a wide variety of oils, solvents, and dyes.
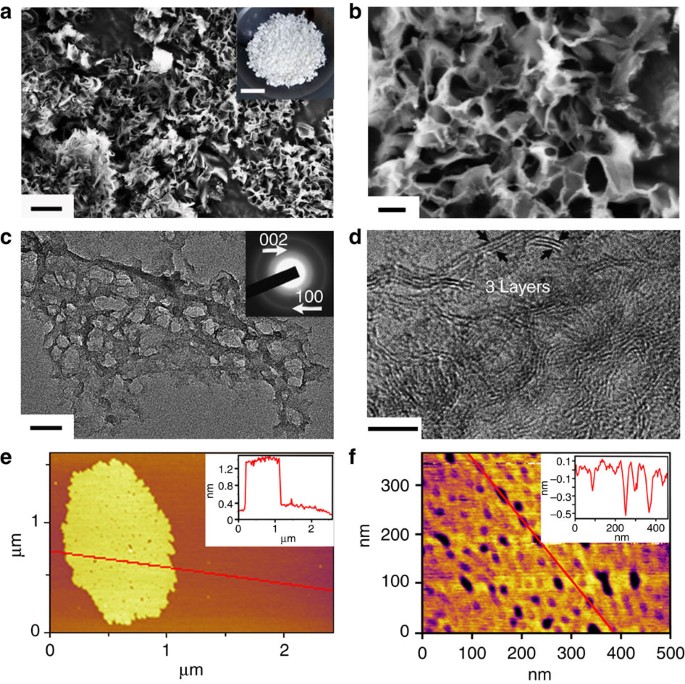
Porous boron nitride for water cleaning
Source: https://media.springernature.com/m685/springer-static/image/art%3A10.1038%2Fncomms2818/MediaObjects/41467_2013_Article_BFncomms2818_Fig1_HTML.jpg
Boron Nitride nanosheets are also known as white graphene. They consist of a few layers of hexagonal Boron Nitride plans and have unique properties compared to graphene.
Boon Nitride-related nanostructures’ polarity and high surface area offer excellent adsorption properties for various substances, from organic pollutants to hydrogen. Because Boron Nitride is made of light elements, it promises high uptake gravimetric capacities.
The nanostructured material can absorb up to 33 times its weight in oils and other organic solvents while repelling water. Because of their oxidation resistance, the saturated Boron Nitride nanosheets can easily be cleaned by heating or burning in the air.
The Boron Nitride has a high adsorption capacity for methyl orange and copper ions. Its maximum removal capacity is 298.3 and 373 mg g-1, at 298 K, respectively. This further shows the potential for porous Boron Nitride nanosheets in water treatment and purification.
Catalysis
Boron Nitride has a wide range of catalytic functions. For use as a solid catalyst, researchers created highly porous turbostratic boron nitride. The Boron Nitride was very weak in base sites and had high activity for the nitroaldol react with high selectivity to Nitroalkene. Their catalytic activity was strongly correlated with the porous structure of boron nitride-catalysts.
Desulfurization of Model Oil
Recent research reported the controlled synthesis of porous Boron Nitride microfibers. It also explored their potential as adsorbents to remove dibenzothiophene from model oil. Correlating the structural characteristics of absorbent Boron Nitride microfibers with their synthesis conditions has helped to understand their growth evolution.
According to the Langmuir isotherm model, prepared Boron Nitride microfibers exhibited a very high adsorption capability for DBT (86mgS g-1) and excellent adsorptive desulfurization performance.
Simple heat treatment can regregeneratee porous Boron Nitride following adsorption. The regenerated adsorption ability is still 83% higher than the initial adsorption. This can be done four times. Thus, porous Boron Nitride microfibers are an excellent adsorbent for sulfur removal from fuels due to their excellent oxidation resistance, chemical inertness, and high sulfur adsorption capacities.
Hydrogen Storage
A study reported synthesizing high-quality microporous/mesoporous Boron Nitride material via a facile two-step approach. The group elimination of organic compounds in a Boron Nitride precursor, melamine-diborate molecular crystalline, was responsible for forming the porous structure.
The ordered pore structure was preserved, and this elimination process did not affect any structural defects. It proved to be highly suitable for hydrogen storage due to its high surface area, pore-volume, and structural defects.
This study shows that the Boron Nitride has a high hydrogen uptake capability, with increased weight adsorption of up to 5.6% at room temperatures and relatively low pressure (3 MPa).